Unit 13 Density 13 1 What is density

















- Slides: 30

Unit 13 Density

13 -1 What is density? Which do you think is heavier, a kilogram of feathers or a kilogram of lead? Which takes up more space, a kilogram of feathers or a kilogram of lead?

13 -1 Density is the mass per unit volume of a substance Lead has a much greater density than feathers, so a kilogram of lead takes up less space than a kilogram of feathers.

13 -1 Units of Density Units of density include units of mass and units of volume Volume can be measured in cubic centimeters (cm 3) Mass can be measured in grams (g) Grams per cubic centimeter is a unit of density (g/cm 3)

13 -1 Density is a basic physical property of all matter Every pure substance has a density that can be measured. For a pure substance, the density is always the same. Density can be used to help identify a material. How could you tell the difference between silver and aluminum?

13 -1 Check 1. Density is the _____ per unit volume of a substance. 2. When a substance has a high density, a large mass fits into a _____ volume. 3. The units of _____ are grams per cubic centimeter. 4. Density is a physical _____ of all matter. 5. The density of a substance is always the _____.

13 -1 Example Problem What is the density of a metal block that has a mass of 750 g and a volume of 55 cm 3? What material is the metal block made out of?

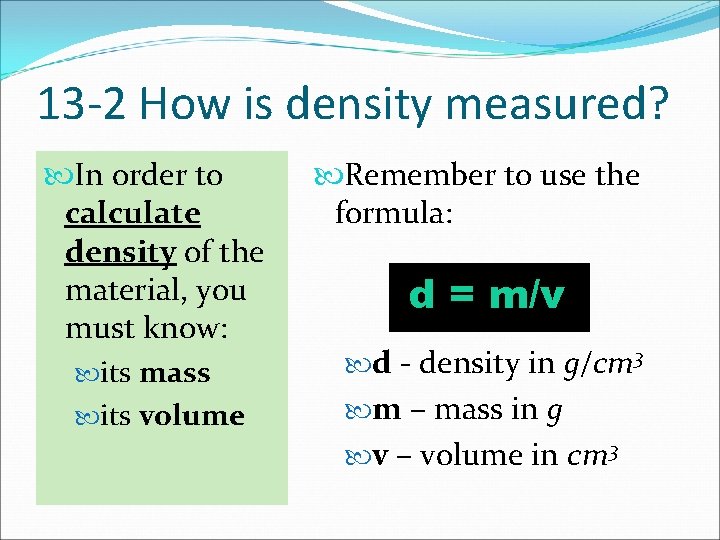
13 -2 How is density measured? In order to calculate density of the material, you must know: its mass its volume Remember to use the formula: d = m/v d - density in g/cm 3 m – mass in g v – volume in cm 3

13 -2 Density of a Liquid You can find the density of a liquid using a graduated cylinder and a balance: 1. Find the mass of the graduated cylinder alone. 2. Find the mass of the graduated cylinder with the liquid in it. 3. What is the mass of the liquid? 4. Read the volume of the liquid on the graduated cylinder. 5. Divide the mass by the volume.

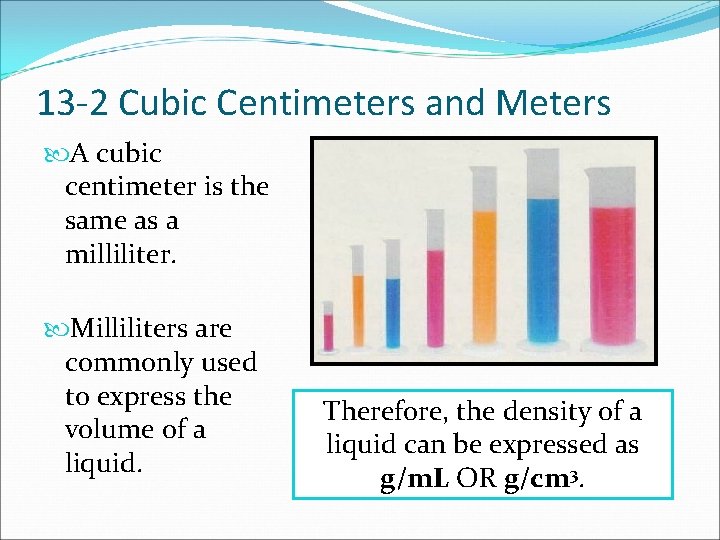
13 -2 Cubic Centimeters and Meters A cubic centimeter is the same as a milliliter. Milliliters are commonly used to express the volume of a liquid. Therefore, the density of a liquid can be expressed as g/m. L OR g/cm 3.

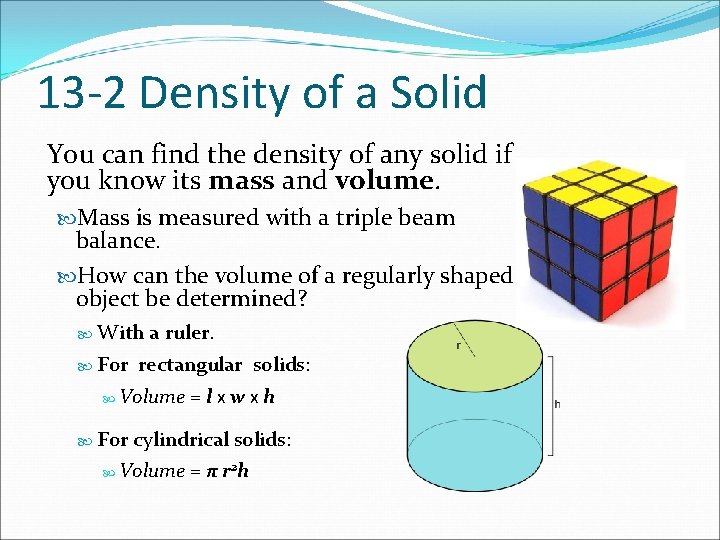
13 -2 Density of a Solid You can find the density of any solid if you know its mass and volume. Mass is measured with a triple beam balance. How can the volume of a regularly shaped object be determined? With a ruler. For rectangular solids: Volume = l x w x h For cylindrical solids: Volume = π r 2 h

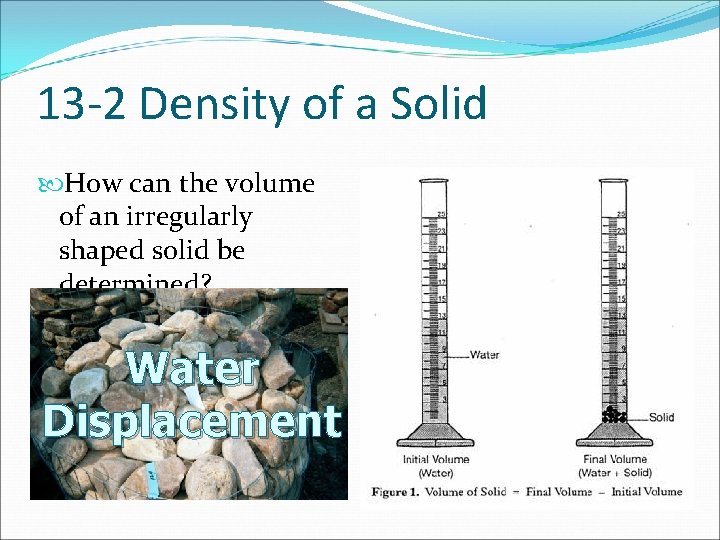
13 -2 Density of a Solid How can the volume of an irregularly shaped solid be determined? Water Displacement

Density Lab You will be working in pairs for this lab. Part of your grade for this lab will be based on the following: Following directions. Proper care of instruments being used. Proper behavior - No fooling around. Good Teamwork. Objectives: 1. You will determine the density of the unknown substance at each station. 2. You will use the table provided to you to determine the identity of each unknown substance. 3. You will answer the analysis questions using your data.

13 -2 Problem If 5 m. L of a liquid have a mass of 10 g, what is the density of the liquid?

13 -2 Problem A bar of a metal measures 2 cm by 20 cm by 10 cm. If the bar has a mass of 1000 g, what is its density?

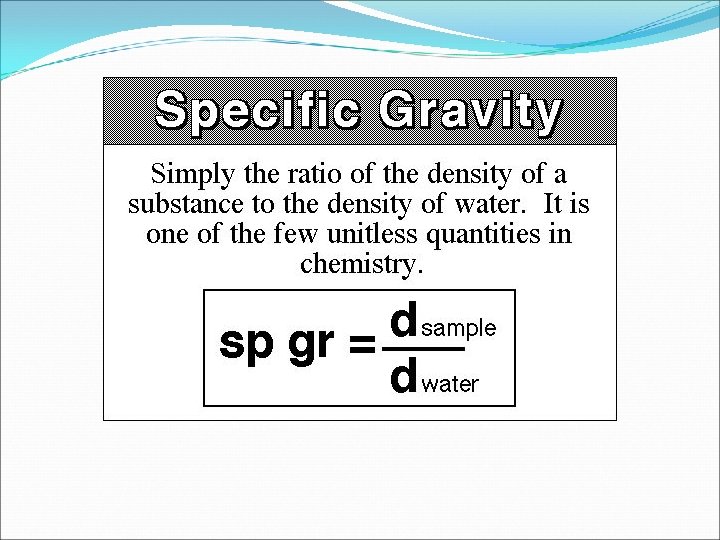
13 -3 What is specific gravity? Compares the density of a substance to the density of water. The density of water is 1 g/cm 3. To find the specific gravity of a substance, divide its density by the density of water. The units cancel out.


13 -3 Uses of Specific Gravity Can be used to identify a substance because the specific gravity of each substance is unique Can be used to check the purity of substances (milk, for example) Can be used to check the amount of battery acid in a car – acid increases the specific gravity of liquid in the battery

13 -3 Measuring Specific Gravity Measured with a device called a hydrometer. A hydrometer is a sealed tube with a weight in the bottom and markings along one side. The hydrometer will float in a liquid – the higher it floats, the higher the specific gravity of the liquid

13 -3 Using a Hydrometer

13 -3 Check Specific gravity compares the density of a substance with the density of _____. Specific gravity has no _____ because the density units cancel out. Specific gravity can be used to check the battery acid in a _____. A hydrometer is a device that can be used to measure the specific gravity of a _____. The _____ at which the hydrometer floats depends on the density of the liquid.

13 -3 Problem In which liquid would a hydrometer float lower, gasoline or corn syrup? Explain.

13 -3 Problems Silver has a density of 10. 5 g/cm 3. What is the specific gravity of silver? Why does specific gravity have no units?

13 -5 What is buoyancy? Buoyancy is the upward force exerted by a gas or liquid. Archimedes’ Principle says that the loss of weight of a body in water equals the weight of the displaced water. He based this on the observation that he felt lighter in water.

13 -5 Buoyancy Gravity An object in water seems lighter… Why? Water exerts an upward force on the object The upward force decreases the downward force of gravity Buoyant Force

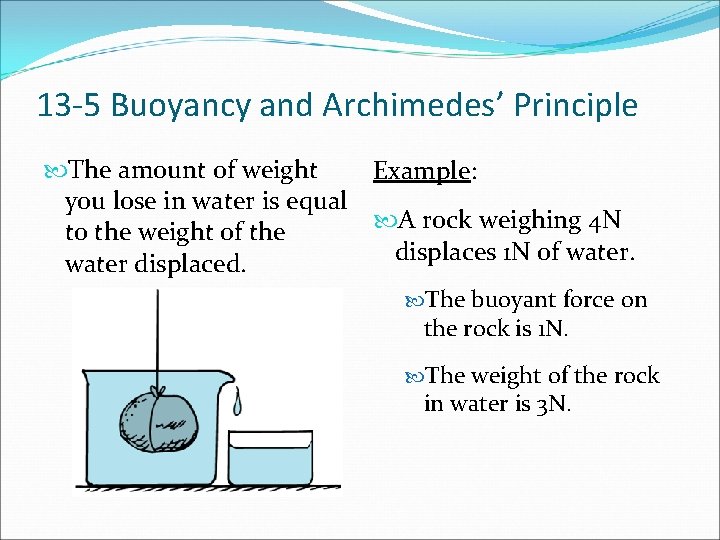
13 -5 Buoyancy and Archimedes’ Principle The amount of weight you lose in water is equal to the weight of the water displaced. Example: A rock weighing 4 N displaces 1 N of water. The buoyant force on the rock is 1 N. The weight of the rock in water is 3 N.

13 -5 Floating Buoyancy explains why some things sink and others float. If an object displaces an amount of water equal to its own weight, then it’s weight in water is zero and it will float.

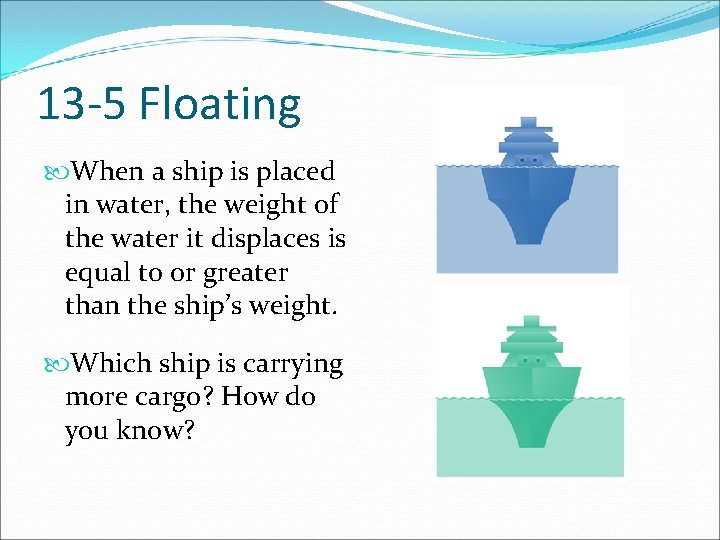
13 -5 Floating When a ship is placed in water, the weight of the water it displaces is equal to or greater than the ship’s weight. Which ship is carrying more cargo? How do you know?

13 -5 Check 1. Buoyancy is the _____ force exerted by a gas or liquid. 2. The buoyant force on an object is equal to the weight of the water it _____. 3. When the buoyant force on an object is equal to or greater than its weight, the object will _____. 4. Buoyancy decreases the downward pull of _____ on an object. 5. A ship floats because the weight of water it displaces is equal to or greater than its own _____.

Test is on Friday! Review Game tomorrow for Bonus Points! The formulas will be provided to you. Bring a calculator!!!! We do not have class on Thursday (due to the half day for parent-teacher conferences). Don’t forget to study for the test.