Density What is density l Density is a













- Slides: 20

Density

What is density? l Density is a comparison of how much matter there is in a certain amount of space.

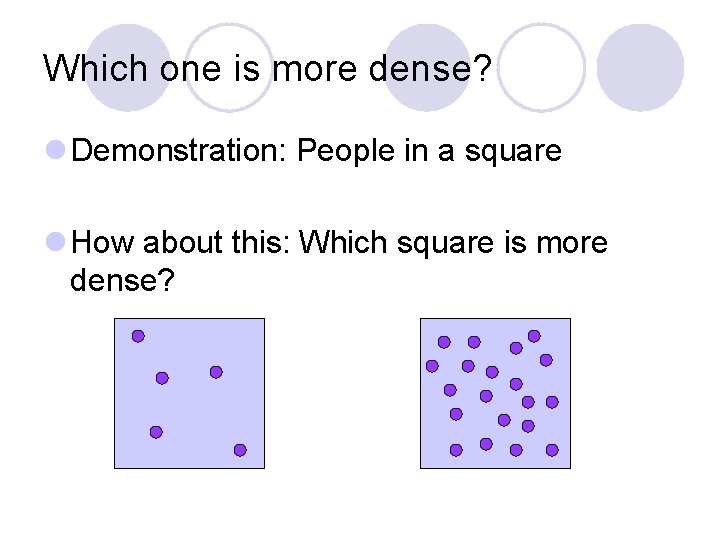
Which one is more dense? l Demonstration: People in a square l How about this: Which square is more dense?

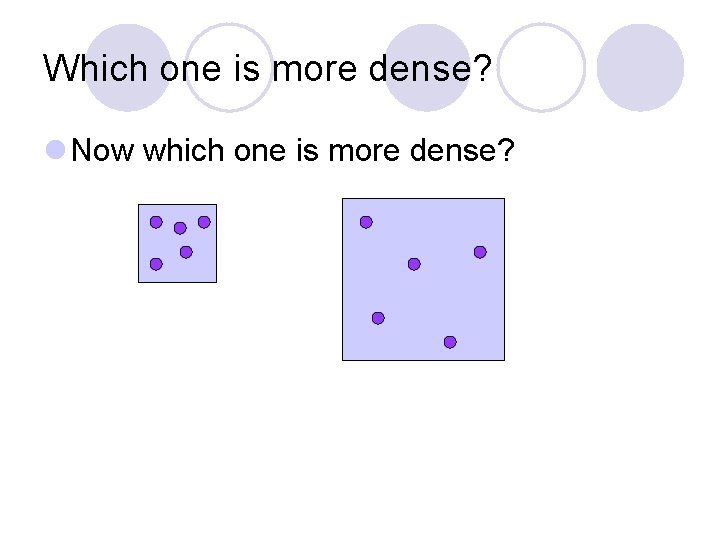
Which one is more dense? l Now which one is more dense?

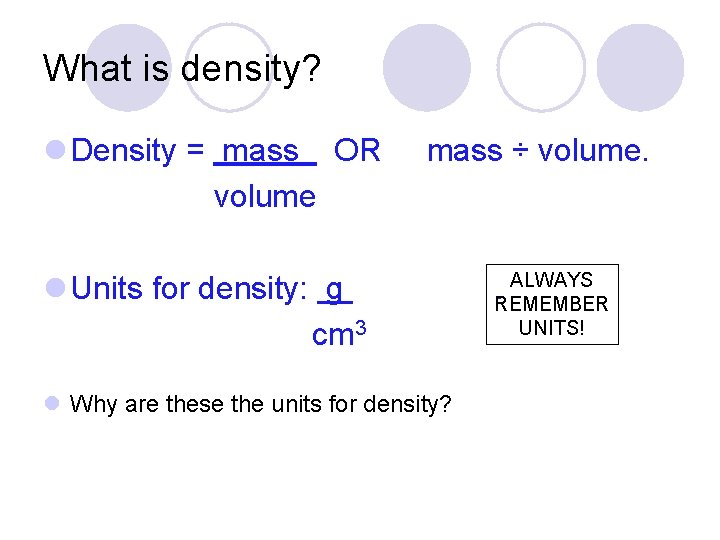
What is density? l Density = mass OR volume mass ÷ volume. l Units for density: g cm 3 l Why are these the units for density? ALWAYS. REMEMBER UNITS!

Let’s try a density problem together l Frank has a paper clip. It has a mass of 9 g and a volume of 3 cm 3. What is its density? l Frank also has an eraser. It has a mass of 3 g, and a volume of 1 cm 3. What is its density?

Work on these problems with your neighbor. l Jack has a rock. The rock has a mass of 6 g and a volume of 3 cm 3. What is the density of the rock? l Jill has a gel pen. The gel pen has a mass of 8 g and a volume of 2 cm 3. What is the density of the pen?

Now, try these on your own. l Alicia has a watch. It has a mass of 4 g and a volume of 2 cm 3. What is the density of the watch? l Mia has a wallet. It has a mass of 15 g and a volume of 5 cm 3. What is the density of the wallet?

Liquid Layers l If you pour together liquids that don’t mix and have different densities, they will form liquid layers. l The liquid with the highest density will be on the bottom. l The liquid with the lowest density will be on the top.

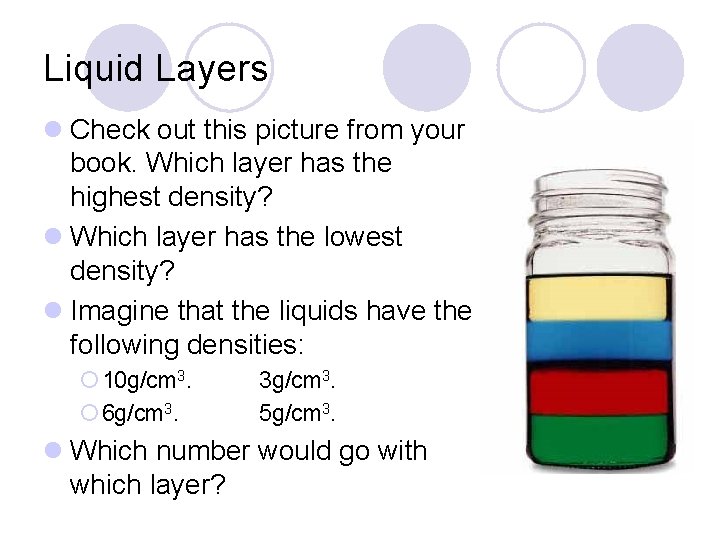
Liquid Layers l Check out this picture from your book. Which layer has the highest density? l Which layer has the lowest density? l Imagine that the liquids have the following densities: ¡ 10 g/cm 3. ¡ 6 g/cm 3. 3 g/cm 3. 5 g/cm 3. l Which number would go with which layer?

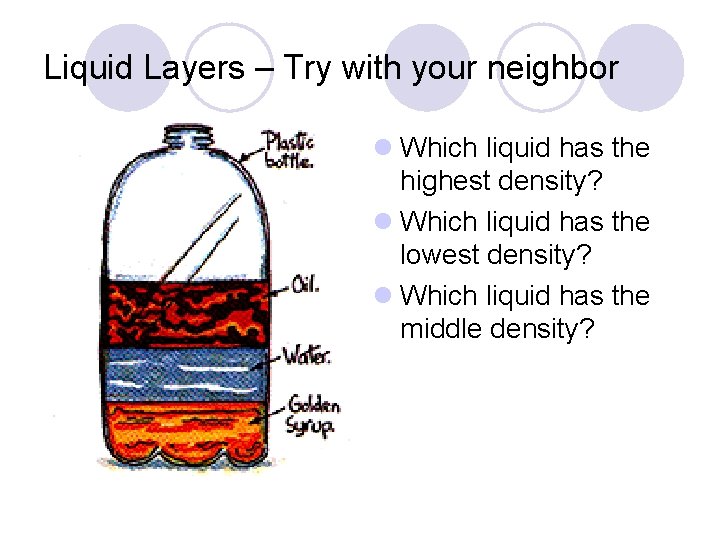
Liquid Layers – Try with your neighbor l Which liquid has the highest density? l Which liquid has the lowest density? l Which liquid has the middle density?

Liquid Layers – Try on your own! l Imagine that the liquids on the right have the following densities: ¡ 15 g/cm 3 ¡ 3 g/cm 3 ¡ 7 g/cm 3 10 g/cm 3 9 g/cm 3 12 g/cm 3 l Match the colors to the correct densities. 3 g/cm 3 7 g/cm 3 9 g/cm 3 10 g/cm 3 12 g/cm 3 15 g/cm 3

Review l What is the formula for density? l What happens if you pour together liquids that have different densities? l Will the liquid on the top have the highest or lowest density? l Will the liquid on the bottom have the highest or lowest density?

Super Scientist Question of the Day l Jake has a book, a ruler, and a balance. l How can Jake find the density of the book with the tools he has?

PG. 2 ON DENSITY Practice A: Volume (L x W x H) a. An object has a length of 3 cm, a width of 2 cm, and a height of 4 cm. Calculate volume. 3 cm x 2 cm x 4 cm = 24 cm 3 b. A cube has sides that measure 5 cm. Calculate volume. 5 cm x 5 cm = 125 cm 3

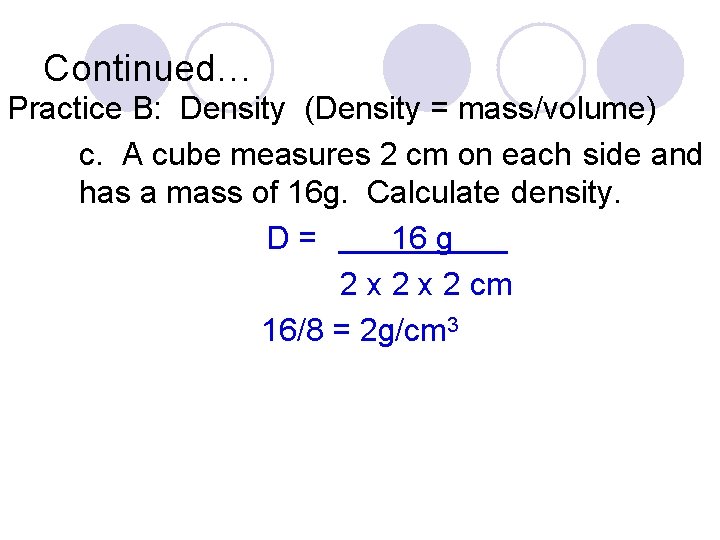
Continued… Practice B: Density (Density = mass/volume) c. A cube measures 2 cm on each side and has a mass of 16 g. Calculate density. D= 16 g 2 x 2 cm 16/8 = 2 g/cm 3


Continued… l Practice c: Density in water ¡Water has density of 1 g/cm 3 ¡Density > Water = sink ¡Density < Water = float d. Will the object in question (e) sink or float in water? SINK 2>1

Density Vocab l Mass – amount of matter that makes up a substance l Volume – amount of space an object takes up ¡(L x W x H) - regularly shaped solid ¡water displacement - irregular solids l(water before object – water after object) l. Find the difference using a graduated cylinder

Density ¡Water has density of 1 g/cm 3 ¡Density > Water = sink ¡Density < Water = float