Density What is density l Density is a


- Slides: 16

Density

What is density? l Density is a comparison of how much matter there is in a certain amount of space.

Density • Density is an INTENSIVE property of matter. - does NOT depend on quantity of matter. • Contrast with EXTENSIVE - depends on quantity of matter. - mass and volume Styrofoam Brick

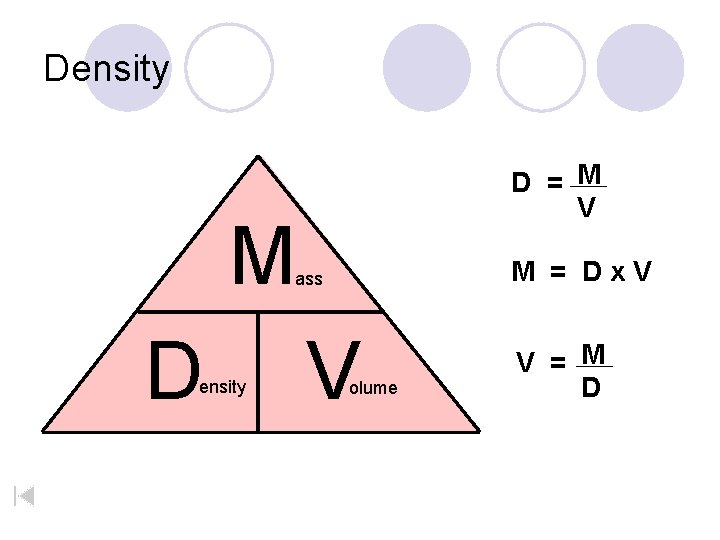
Density D = M V M M = Dx. V ass D ensity V olume V = M D

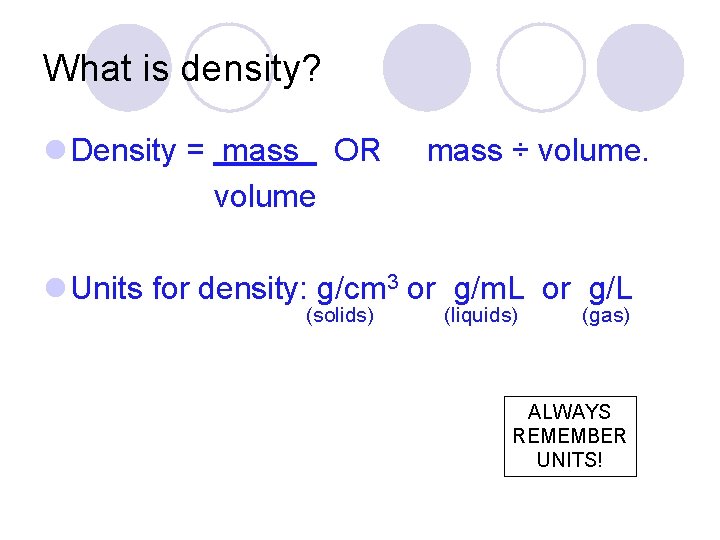
What is density? l Density = mass OR volume mass ÷ volume. l Units for density: g/cm 3 or g/m. L or g/L (solids) (liquids) (gas) ALWAYS REMEMBER UNITS!

Mass l Mass is the amount of matter an object contains l We find mass by using a balance l Mass is usually measured in grams (g)

Volume l Volume: the amount of space an object occupies l Volume of a regular solid (squares and rectangles): ¡length x width x height ¡cm x cm = cm 3 Metric Units for volume 1 liter (L) = 1000 milliliters (m. L) 1 milliliter (m. L) = 1 cm 3 (or ft 3) = 1 gram*

Volume cont. l Volume of an irregular solid (like a rock) ¡Water Displacement Method l. Subtract (final volume – initial volume) to find the volume of the object l. How could we do this? ? l. The volume can be expressed in cm 3 since 1 m. L of water = 1 cm 3 l Volume of a liquid ¡Measured using a graduated cylinder

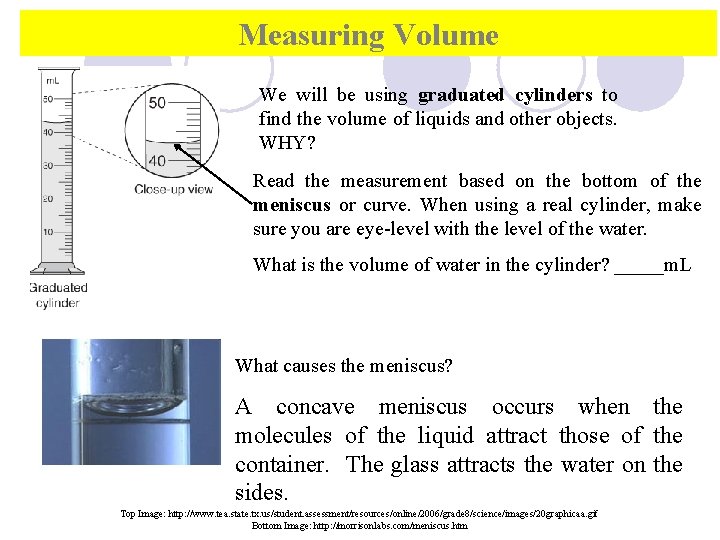
Measuring Volume We will be using graduated cylinders to find the volume of liquids and other objects. WHY? Read the measurement based on the bottom of the meniscus or curve. When using a real cylinder, make sure you are eye-level with the level of the water. What is the volume of water in the cylinder? _____m. L What causes the meniscus? A concave meniscus occurs when the molecules of the liquid attract those of the container. The glass attracts the water on the sides. Top Image: http: //www. tea. state. tx. us/student. assessment/resources/online/2006/grade 8/science/images/20 graphicaa. gif Bottom Image: http: //morrisonlabs. com/meniscus. htm

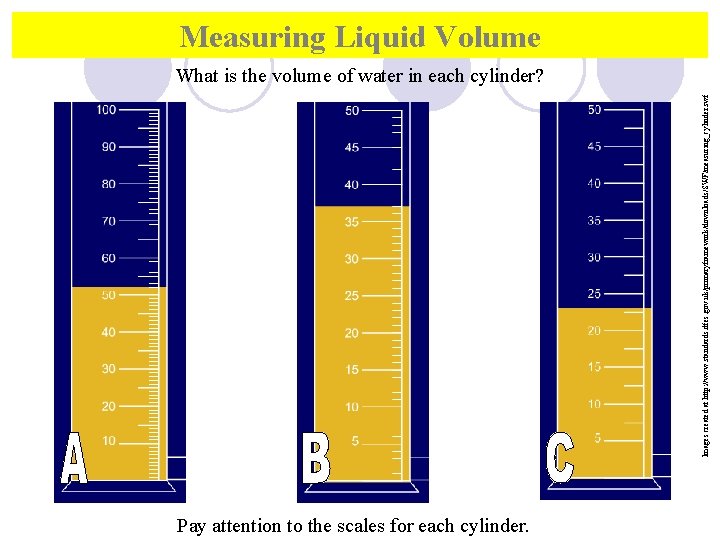
Measuring Liquid Volume Images created at http: //www. standards. dfes. gov. uk/primaryframework/downloads/SWF/measuring_cylinder. swf What is the volume of water in each cylinder? Pay attention to the scales for each cylinder.

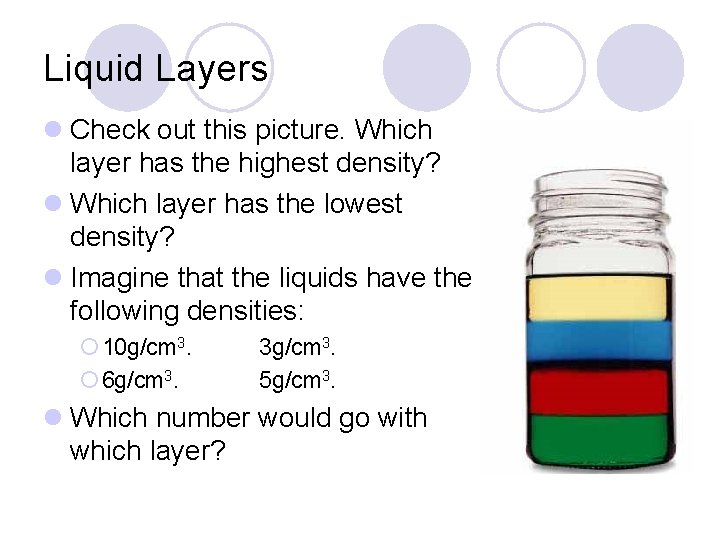
Liquid Layers l Check out this picture. Which layer has the highest density? l Which layer has the lowest density? l Imagine that the liquids have the following densities: ¡ 10 g/cm 3. ¡ 6 g/cm 3. 3 g/cm 3. 5 g/cm 3. l Which number would go with which layer?

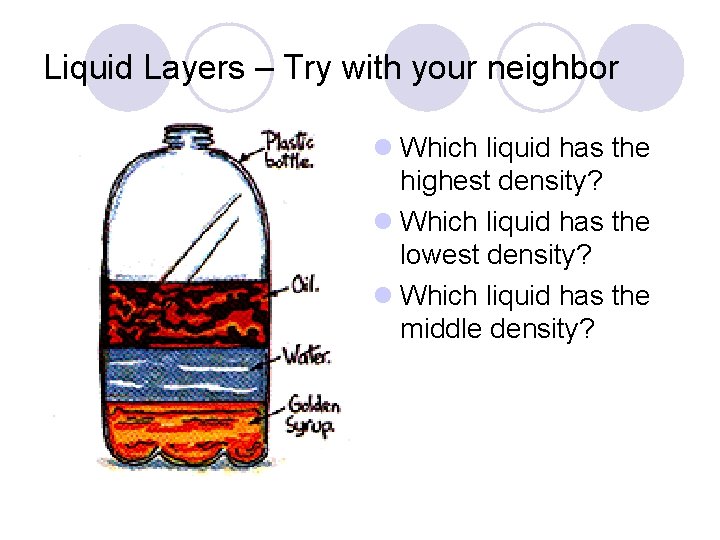
Liquid Layers – Try with your neighbor l Which liquid has the highest density? l Which liquid has the lowest density? l Which liquid has the middle density?

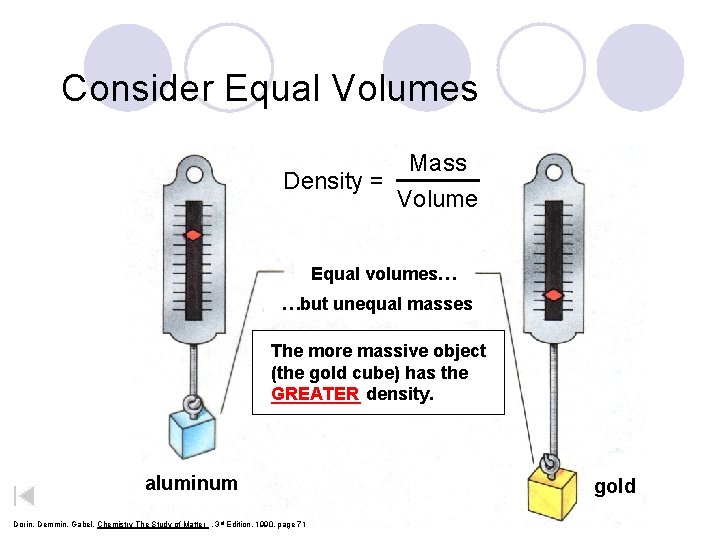
Consider Equal Volumes Mass Density = Volume Equal volumes… …but unequal masses The more massive object (the gold cube) has the GREATER _____ density. aluminum Dorin, Demmin, Gabel, Chemistry The Study of Matter , 3 rd Edition, 1990, page 71 gold

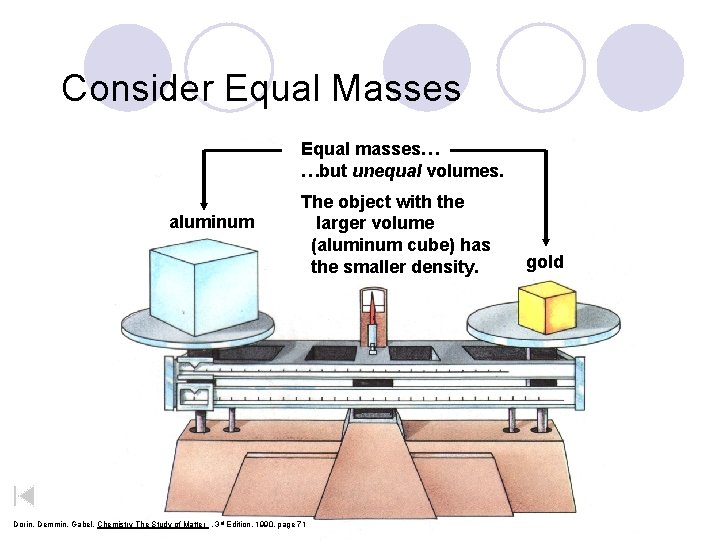
Consider Equal Masses Equal masses… …but unequal volumes. aluminum The object with the larger volume (aluminum cube) has the smaller density. Dorin, Demmin, Gabel, Chemistry The Study of Matter , 3 rd Edition, 1990, page 71 gold

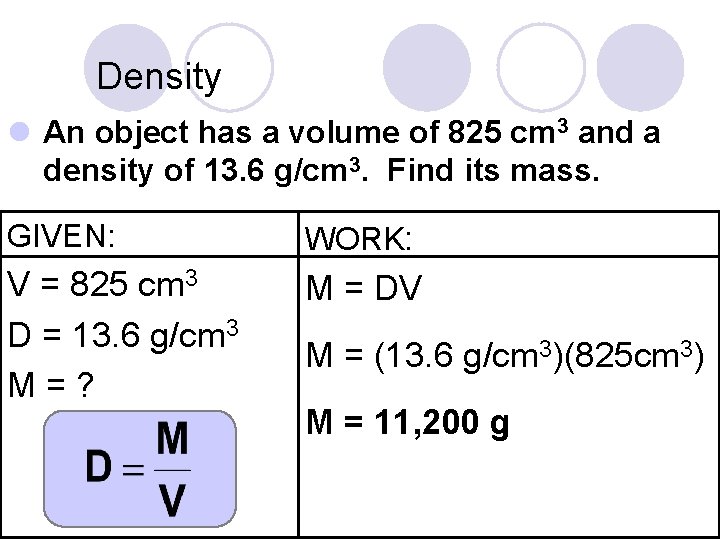
Density l An object has a volume of 825 cm 3 and a density of 13. 6 g/cm 3. Find its mass. GIVEN: WORK: V = 825 cm 3 D = 13. 6 g/cm 3 M=? M = DV M = (13. 6 g/cm 3)(825 cm 3) M = 11, 200 g

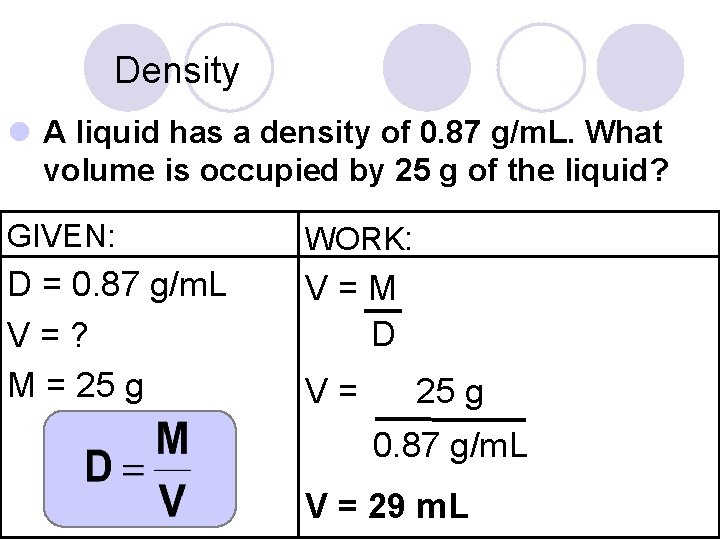
Density l A liquid has a density of 0. 87 g/m. L. What volume is occupied by 25 g of the liquid? GIVEN: WORK: D = 0. 87 g/m. L V=? M = 25 g V=M D V= 25 g 0. 87 g/m. L V = 29 m. L