DENSITY Concepts to explain DENSITY Density the Mass









- Slides: 16

DENSITY

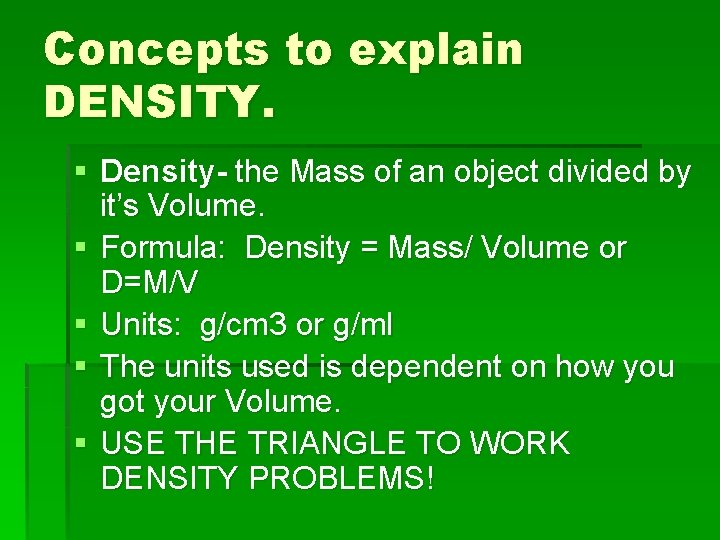
Concepts to explain DENSITY. § Density- the Mass of an object divided by it’s Volume. § Formula: Density = Mass/ Volume or D=M/V § Units: g/cm 3 or g/ml § The units used is dependent on how you got your Volume. § USE THE TRIANGLE TO WORK DENSITY PROBLEMS!

Sample Problems § Raven measured the Mass of a rock on the Triple Beam Balance. She found it to be 5 grams. She then measured the Volume in a Graduated Cylinder. Her initial Volume was 20 ml and her final Volume was 24 ml. What is the Density of the Rock? § D=? M=5 g V=4 ml D=M/V=5 g/4 ml=1. 25 g/ml

Things to Know § If an object has a Density GREATER THAN 1 g/ml or 1 g/cm 3, then it will SINK in Water! § If an object has a Density LESS THAN 1 g/ml or 1 g/cm 3, then it will FLOAT in Water. § Density is a characteristic material property; thus the Density of two objects of the same material is always the same even if the masses of the two objects are different.

Problems for You to Master § A wooden cube is measured at 2 cm on one side. It has a mass of 4 g. What is it’s Density and what will it do in H 2 O? § A marbles’ mass is 10 g and when it’s volume was measured the initial volume was 25 ml with a final volume of 32 ml. Calculate the Density and describe what will happen when placed in water?

Density Quiz § § D=? M=6 g V=3 ml M=? D=5 g/cm 3 V=5 cm 3 V=? D=. 75 g/ml M=2 g Find the mass of an object with a Density of 1. 5 g/cm 3 and a Volume of 2 cm 3. § What is the Density of a steel ball that has a mass of 20 g. It was placed in a graduated cylinder with an initial Volume of 20 ml and a final Volume of 35 ml?

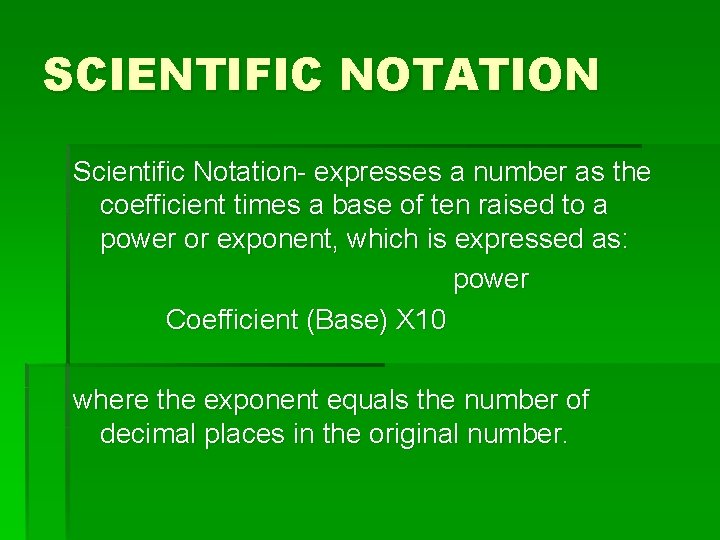
SCIENTIFIC NOTATION Scientific Notation- expresses a number as the coefficient times a base of ten raised to a power or exponent, which is expressed as: power Coefficient (Base) X 10 where the exponent equals the number of decimal places in the original number.

Use of Scientific Notation § Scientific Notation simplifies very large or very small numbers that have many zeros. These large or small numbers are said to be in STANDARD FORM. Example: 5, 947, 398, 021 Km (Large). 0000002963 dg (Small)

Writing in Scientific Notation In the first example; 5, 947, 398, 021 you have to place the decimal behind the first whole number (coefficient) to get 5. 94, next the base is ALWAYS times ten (X 10), and the exponent (power) represents the number of places the decimal was moved to get the base. The result should be as follows: 9 5. 94 X 10

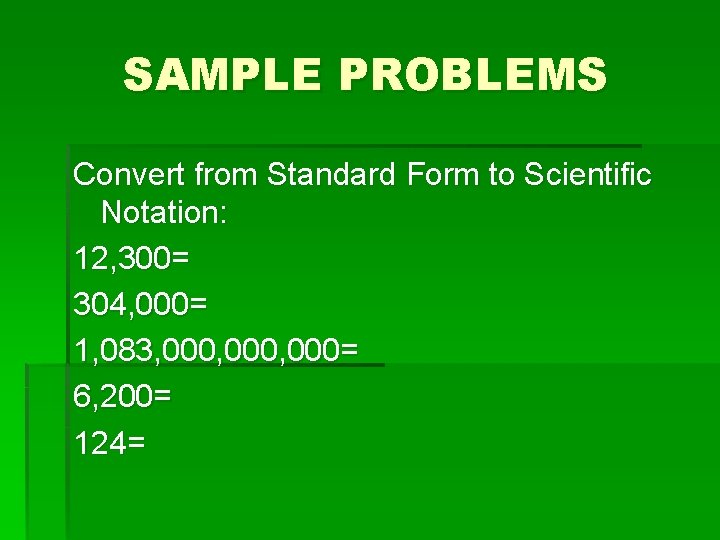
SAMPLE PROBLEMS Convert from Standard Form to Scientific Notation: 12, 300= 304, 000= 1, 083, 000, 000= 6, 200= 124=

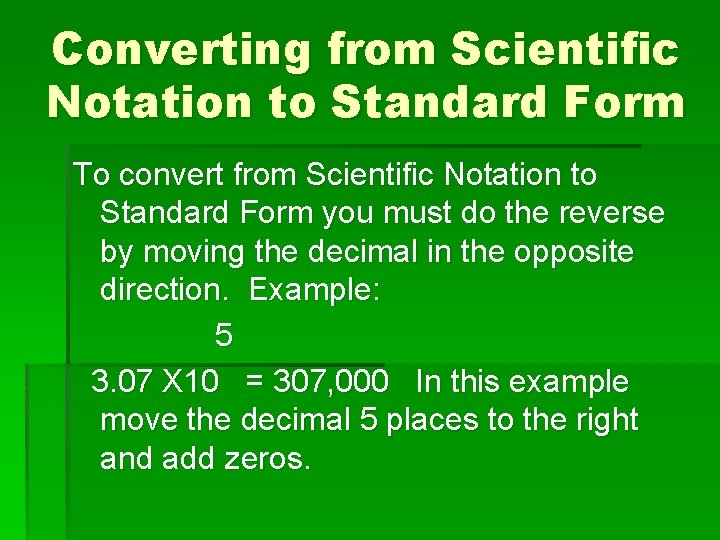
Converting from Scientific Notation to Standard Form To convert from Scientific Notation to Standard Form you must do the reverse by moving the decimal in the opposite direction. Example: 5 3. 07 X 10 = 307, 000 In this example move the decimal 5 places to the right and add zeros.

Small Numbers and Scientific Notation If the Standard Form number is LESS THAN 1 (one), then you must use a NEGATIVE exponent (power). All other rules still apply with the exception of the direction that the decimal is moved. Example: -7. 0000002963 = 2. 96 X 10 Reverse the process when going from Scientific Notation to Standard Form.

More Sample Problems Working with small numbers: . 00047 =. 000000100045 =. 035400 = -5 2. 3 X 10 = -3 5. 48 X 10 =

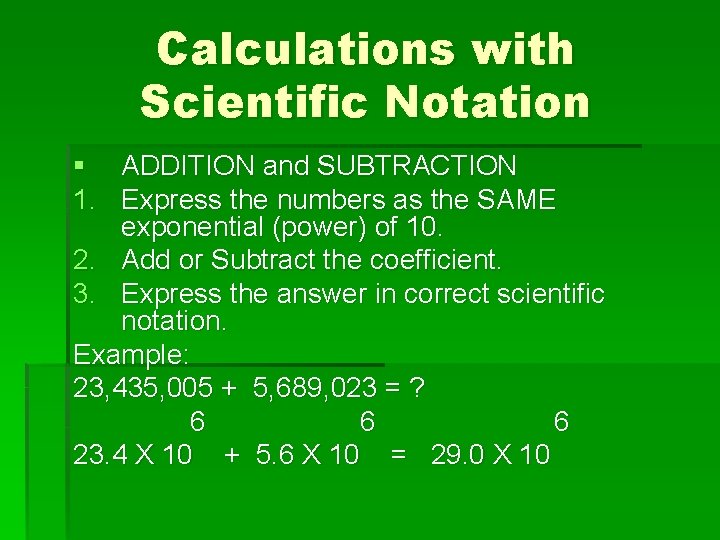
Calculations with Scientific Notation § ADDITION and SUBTRACTION 1. Express the numbers as the SAME exponential (power) of 10. 2. Add or Subtract the coefficient. 3. Express the answer in correct scientific notation. Example: 23, 435, 005 + 5, 689, 023 = ? 6 6 6 23. 4 X 10 + 5. 6 X 10 = 29. 0 X 10

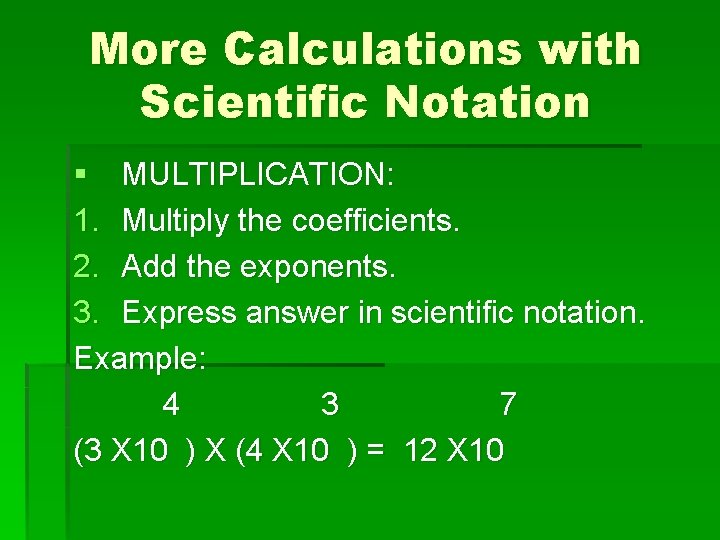
More Calculations with Scientific Notation § MULTIPLICATION: 1. Multiply the coefficients. 2. Add the exponents. 3. Express answer in scientific notation. Example: 4 3 7 (3 X 10 ) X (4 X 10 ) = 12 X 10

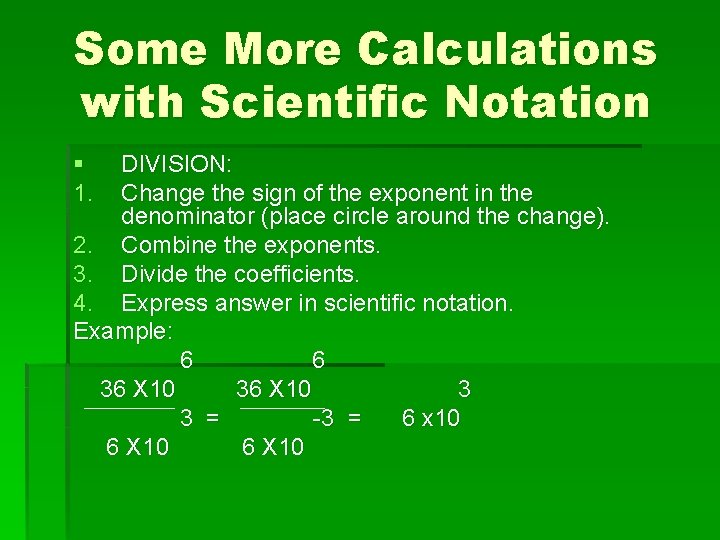
Some More Calculations with Scientific Notation § 1. DIVISION: Change the sign of the exponent in the denominator (place circle around the change). 2. Combine the exponents. 3. Divide the coefficients. 4. Express answer in scientific notation. Example: 6 6 36 X 10 3 3 = -3 = 6 x 10 6 X 10