Mass Spectrometry Background o Mass spectrometry Mass Spec
Mass Spectrometry

Background o Mass spectrometry (Mass Spec or MS) uses high energy electrons to break a molecule into fragments. -이온화, 분자의 분해된 조각 o Separation and analysis of the fragments provides information about: n Molecular weight n Structure -질량대 전하비(mass-to-charge rato) -분자질량, 분자구조 예측

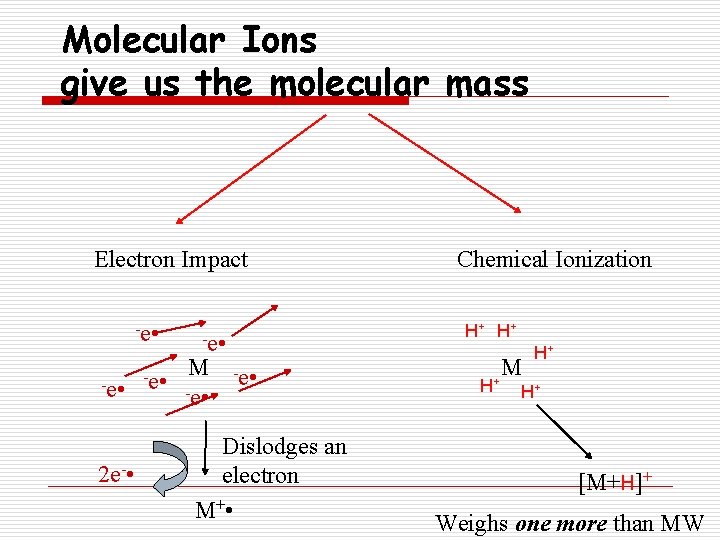
Molecular Ions give us the molecular mass Electron Impact -e • 2 e- • -e • H+ H+ -e • M -e • Chemical Ionization -e • Dislodges an electron M+ • H+ M H+ H+ [M+H]+ Weighs one more than MW
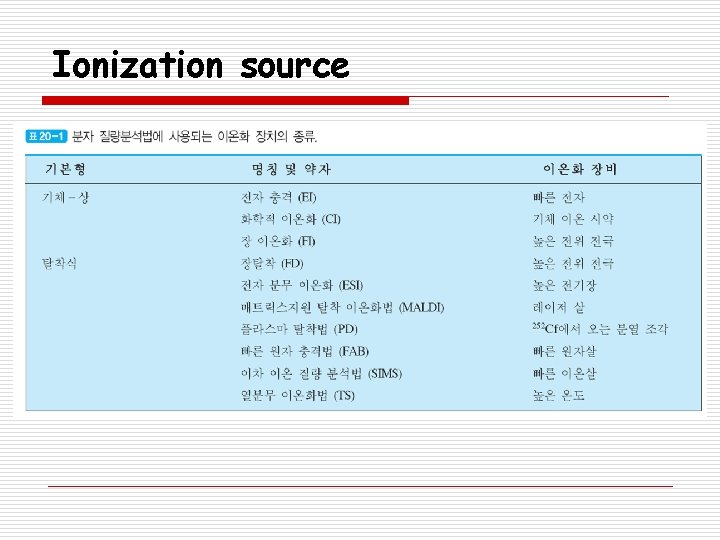
Ionization source
![Fragmentation EI [M·]+ A+ + B· (neutral) or B+ + A· Better carbocation wins Fragmentation EI [M·]+ A+ + B· (neutral) or B+ + A· Better carbocation wins](http://slidetodoc.com/presentation_image_h/e6d6f75f3da73e5256afd0a14380ade0/image-6.jpg)
Fragmentation EI [M·]+ A+ + B· (neutral) or B+ + A· Better carbocation wins and predominates (“Stevenson’s Rule”) CI [M+H]+ PH+ + N (neutral) The “Even Electron Rule” dictates that even (non-radical) ions will not fragment to give two radicals (pos • + neutral • ) (CI)

Fragmentation
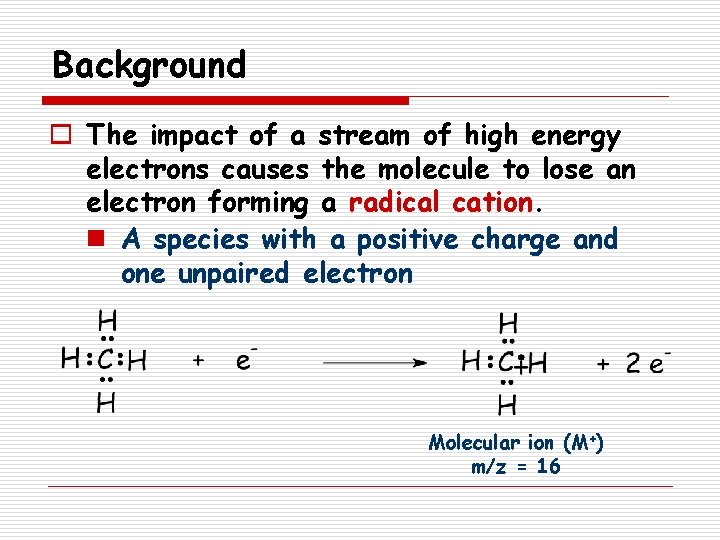
Background o The impact of a stream of high energy electrons causes the molecule to lose an electron forming a radical cation. n A species with a positive charge and one unpaired electron Molecular ion (M+) m/z = 16
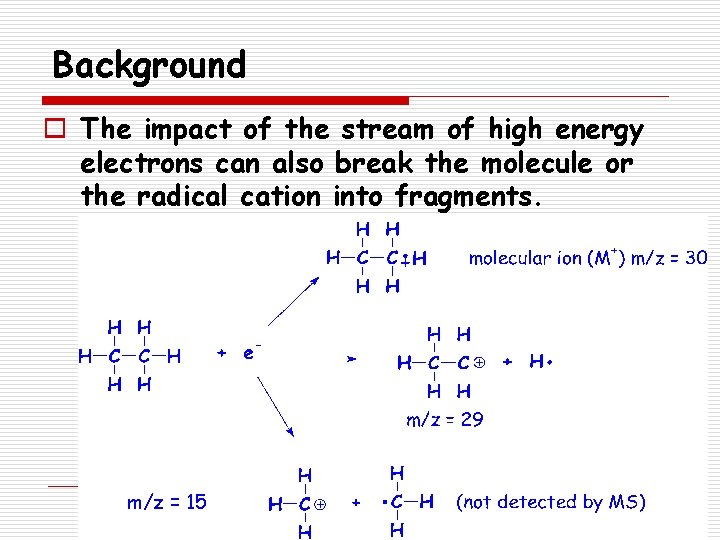
Background o The impact of the stream of high energy electrons can also break the molecule or the radical cation into fragments. m/z = 15

Background o Molecular ion (parent ion): n The radical cation corresponding to the mass of the original molecule o The molecular ion is usually the highest mass in the spectrum n Some exceptions w/specific isotopes n Some molecular ion peaks are absent.
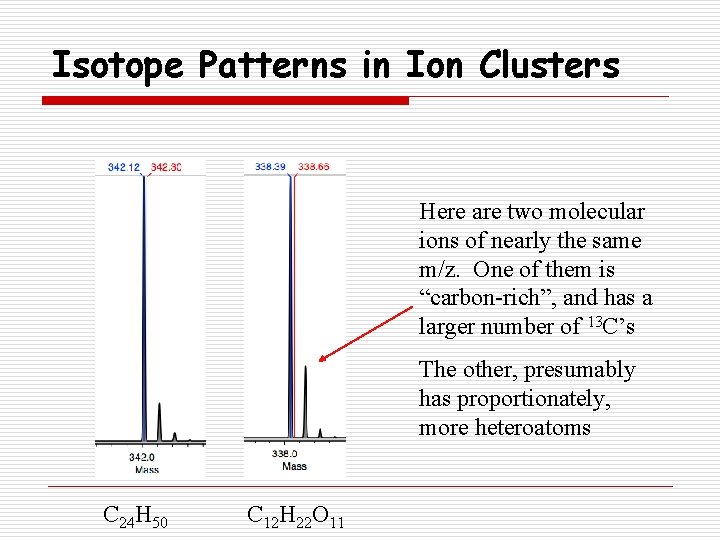
Isotope Patterns in Ion Clusters Here are two molecular ions of nearly the same m/z. One of them is “carbon-rich”, and has a larger number of 13 C’s The other, presumably has proportionately, more heteroatoms C 24 H 50 C 12 H 22 O 11
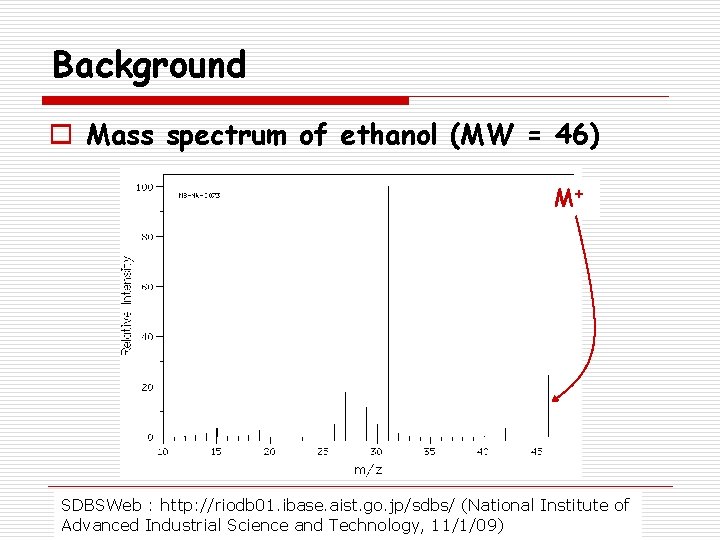
Background o Mass spectrum of ethanol (MW = 46) M+ SDBSWeb : http: //riodb 01. ibase. aist. go. jp/sdbs/ (National Institute of Advanced Industrial Science and Technology, 11/1/09)
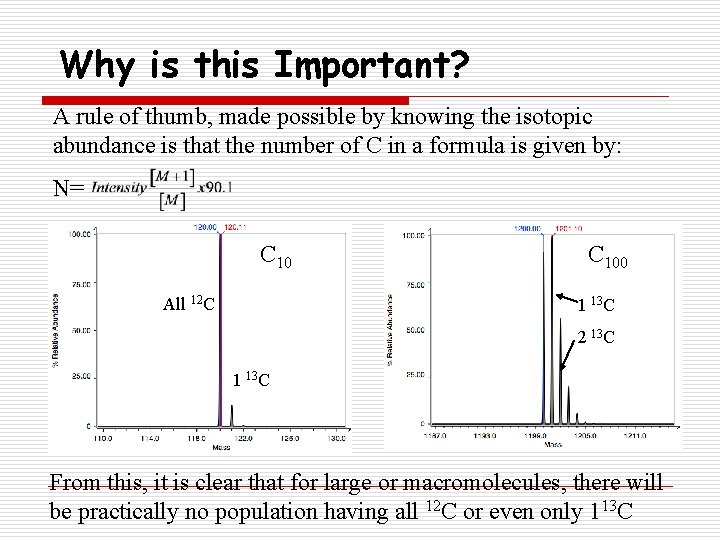
Why is this Important? A rule of thumb, made possible by knowing the isotopic abundance is that the number of C in a formula is given by: N= C 10 All 12 C C 100 1 13 C 2 13 C 1 13 C From this, it is clear that for large or macromolecules, there will be practically no population having all 12 C or even only 113 C

The “Nitrogen Rule” • Molecules containing atoms limited to C, H, O, N, S, X, P of even-numbered molecular weight contain either NO nitrogen or an even number of N • This is true as well for radicals as well. • Not true for pre-charged, e. g. quats, (rule inverts) or radical cations. • In the case of Chemical Ionization, where [M+H]+ is observed, need to subtract 1, then apply nitrogen rule. • Example, if we know a compound is free of nitrogen and gives an ion at m/z=201, then that peak cannot be the molecular ion.
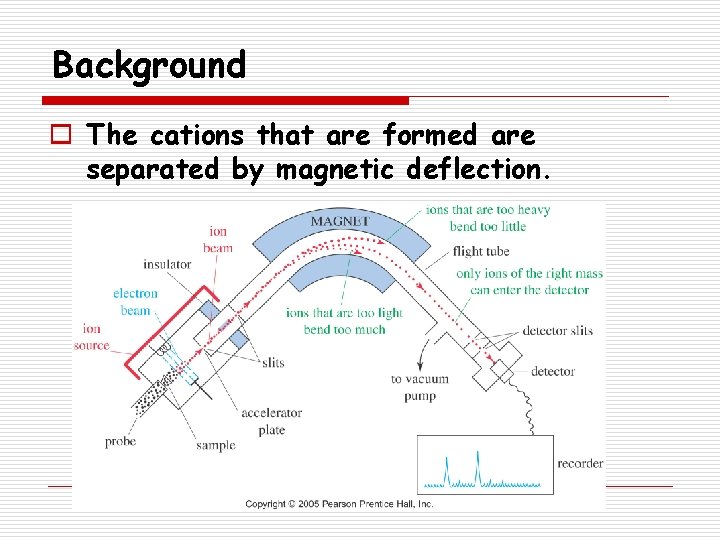
Background o The cations that are formed are separated by magnetic deflection.






Background o Only cations are detected. n Radicals are “invisible” in MS. o The amount of deflection observed depends on the mass to charge ratio (m/z). n Most cations formed have a charge of +1 so the amount of deflection observed is usually dependent on the mass of the ion.

Background o The resulting mass spectrum is a graph of the mass of each cation vs. its relative abundance. o The peaks are assigned an abundance as a percentage of the base peak. n the most intense peak in the spectrum o The base peak is not necessarily the same as the parent ion peak.

Background The mass spectrum of ethanol base peak M+ SDBSWeb : http: //riodb 01. ibase. aist. go. jp/sdbs/ (National Institute of Advanced Industrial Science and Technology, 11/1/09)
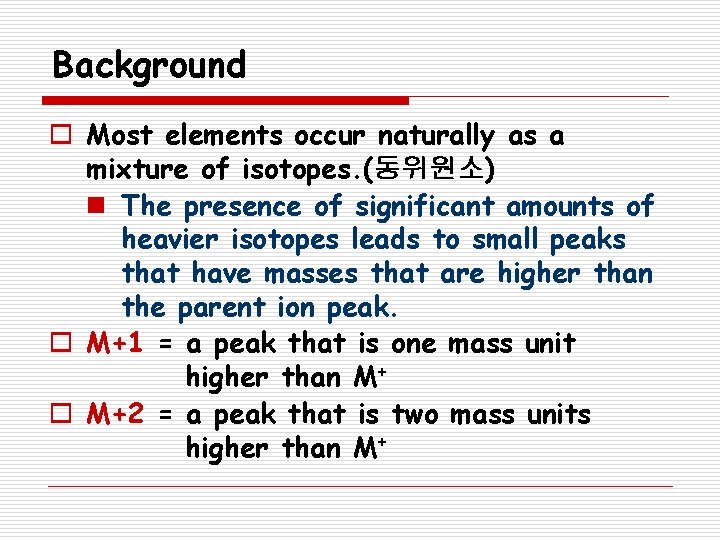
Background o Most elements occur naturally as a mixture of isotopes. (동위원소) n The presence of significant amounts of heavier isotopes leads to small peaks that have masses that are higher than the parent ion peak. o M+1 = a peak that is one mass unit higher than M+ o M+2 = a peak that is two mass units higher than M+
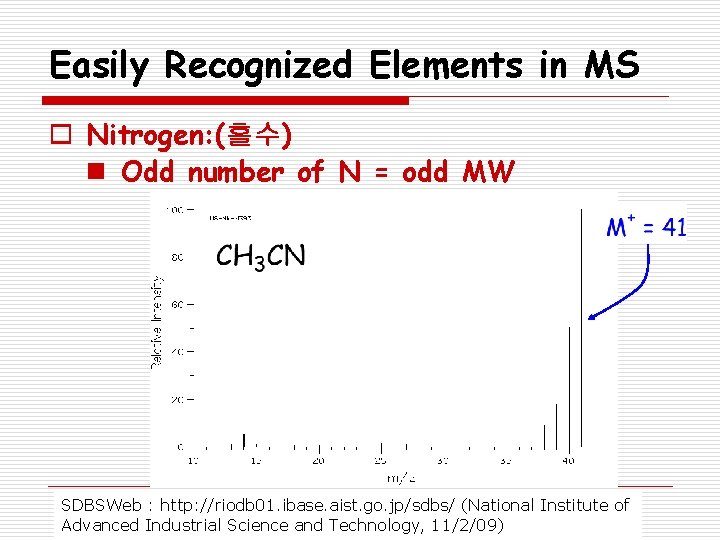
Easily Recognized Elements in MS o Nitrogen: (홀수) n Odd number of N = odd MW SDBSWeb : http: //riodb 01. ibase. aist. go. jp/sdbs/ (National Institute of Advanced Industrial Science and Technology, 11/2/09)
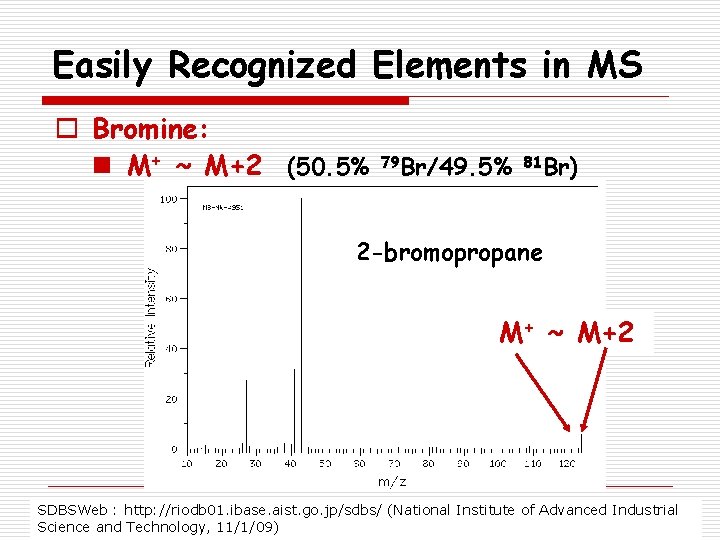
Easily Recognized Elements in MS o Bromine: n M+ ~ M+2 (50. 5% 79 Br/49. 5% 81 Br) 2 -bromopropane M+ ~ M+2 SDBSWeb : http: //riodb 01. ibase. aist. go. jp/sdbs/ (National Institute of Advanced Industrial Science and Technology, 11/1/09)

Easily Recognized Elements in MS o Chlorine: n M+2 is ~ 1/3 as large as M+ M+ M+2 SDBSWeb : http: //riodb 01. ibase. aist. go. jp/sdbs/ (National Institute of Advanced Industrial Science and Technology, 11/2/09)
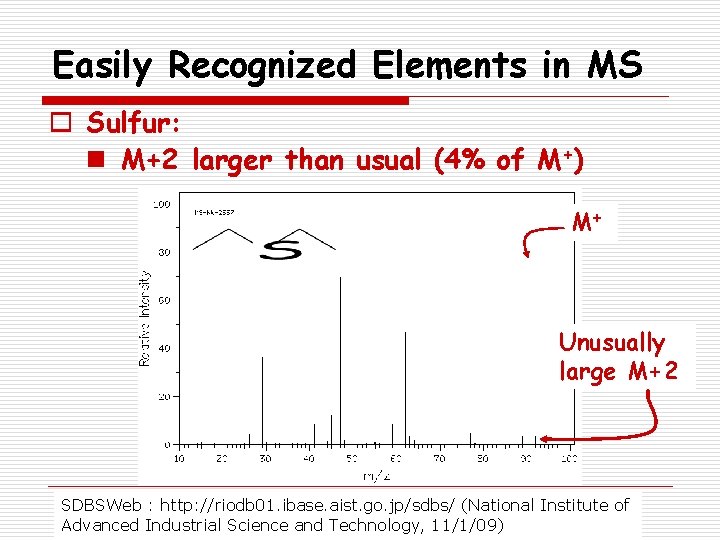
Easily Recognized Elements in MS o Sulfur: n M+2 larger than usual (4% of M+) M+ Unusually large M+2 SDBSWeb : http: //riodb 01. ibase. aist. go. jp/sdbs/ (National Institute of Advanced Industrial Science and Technology, 11/1/09)
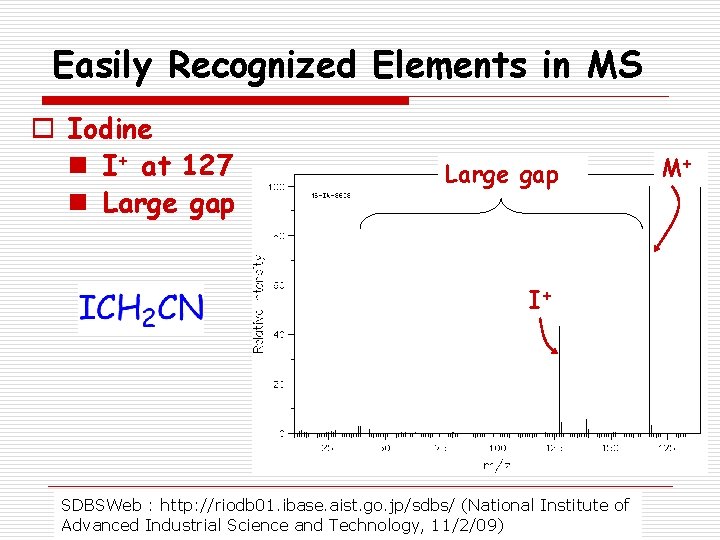
Easily Recognized Elements in MS o Iodine n I+ at 127 n Large gap I+ SDBSWeb : http: //riodb 01. ibase. aist. go. jp/sdbs/ (National Institute of Advanced Industrial Science and Technology, 11/2/09) M+

Fragmentation Patterns o The impact of the stream of high energy electrons often breaks the molecule into fragments, commonly a cation and a radical. n Bonds break to give the most stable cation. n Stability of the radical is less important.

Fragmentation Patterns o Alkanes n Fragmentation often alkyl groups: o Loss of methyl o Loss of propyl o Loss of butyl splits off simple M+ M+ - 15 29 43 57 n Branched alkanes tend to fragment forming the most stable carbocations.
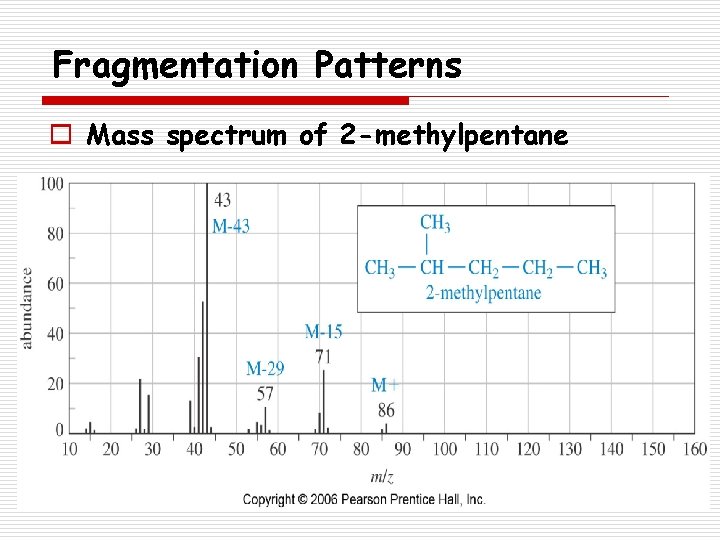
Fragmentation Patterns o Mass spectrum of 2 -methylpentane
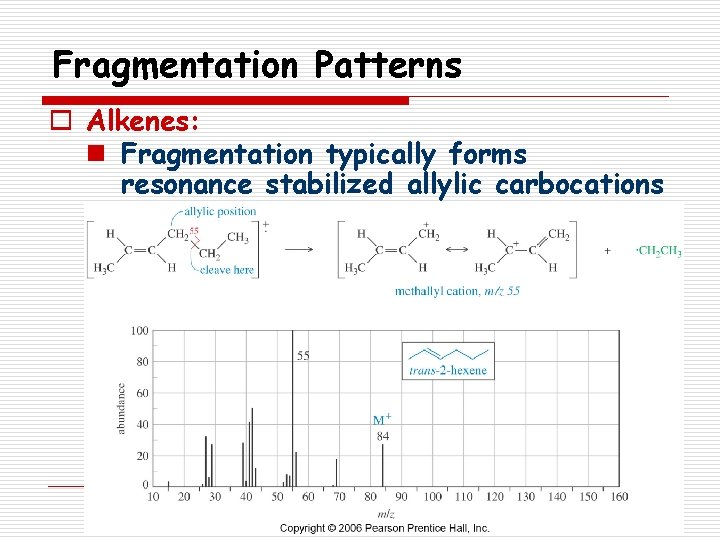
Fragmentation Patterns o Alkenes: n Fragmentation typically forms resonance stabilized allylic carbocations

Fragmentation Patterns o Aromatics: n Fragment at the benzylic carbon, forming a resonance stabilized benzylic carbocation (which rearranges to the tropylium ion) M+

Fragmentation Patterns Aromatics may also have a peak at m/z = 77 for the benzene ring. 77 M+ = 123
Fragmentation Patterns o Alcohols n Fragment easily resulting in very small or missing parent ion peak n May lose hydroxyl radical or water o M+ - 17 or M+ - 18 n Commonly lose an alkyl group attached to the carbinol carbon forming an oxonium ion. o 1 o alcohol usually has prominent peak at m/z = 31 corresponding to H 2 C=OH+
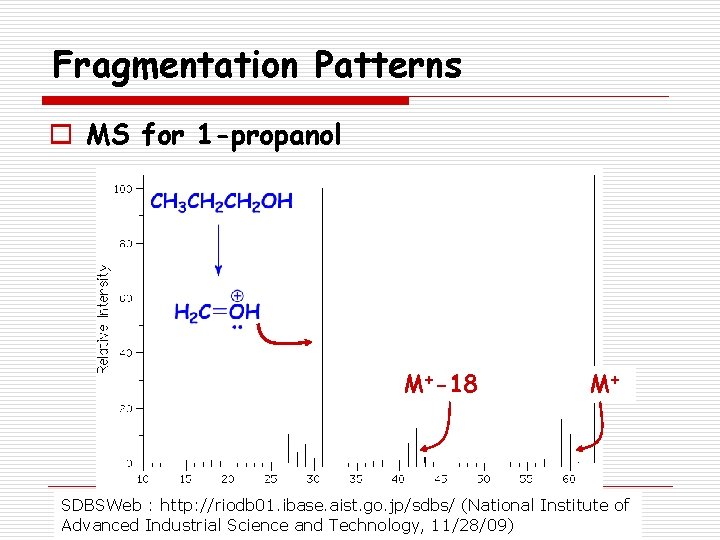
Fragmentation Patterns o MS for 1 -propanol M+-18 M+ SDBSWeb : http: //riodb 01. ibase. aist. go. jp/sdbs/ (National Institute of Advanced Industrial Science and Technology, 11/28/09)

Fragmentation Patterns o Amines n Odd M+ (assuming an odd number of nitrogens are present) n a-cleavage dominates forming an iminium ion

Fragmentation Patterns

Fragmentation Patterns o Ethers n a-cleavage forming oxonium ion n Loss of alkyl group forming a carbocation

Fragmentation Patterns MS of diethylether (CH 3 CH 2 OCH 2 CH 3)

Fragmentation Patterns o Aldehydes (RCHO) n Fragmentation may form acylium ion n Common fragments: o M+ - 1 for o M+ - 29 for

Fragmentation Patterns o MS for hydrocinnamaldehyde 91 M+ = 134 105 SDBSWeb : http: //riodb 01. ibase. aist. go. jp/sdbs/ (National Institute of Advanced Industrial Science and Technology, 11/28/09)

Fragmentation Patterns o Ketones n Fragmentation leads to formation of acylium ion: o Loss of R forming o Loss of R’ forming

Fragmentation Patterns o MS for 2 -pentanone M+ SDBSWeb : http: //riodb 01. ibase. aist. go. jp/sdbs/ (National Institute of Advanced Industrial Science and Technology, 11/28/09)

Fragmentation Patterns o Esters (RCO 2 R’) n Common fragmentation patterns include: o Loss of OR’ n peak at M+ - OR’ o Loss of R’ n peak at M+ - R’
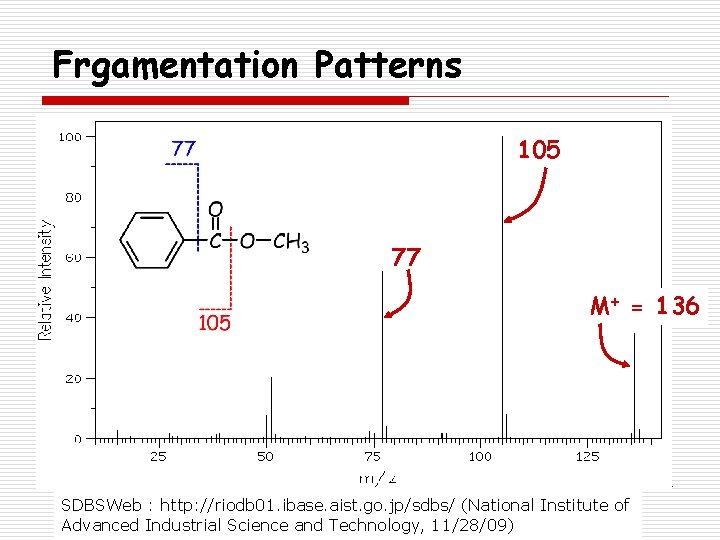
Frgamentation Patterns 105 77 M+ = 136 SDBSWeb : http: //riodb 01. ibase. aist. go. jp/sdbs/ (National Institute of Advanced Industrial Science and Technology, 11/28/09)
Rule of Thirteen o The “Rule of Thirteen” can be used to identify possible molecular formulas for an unknown hydrocarbon, Cn. Hm. n Step 1: n = M+/13 (integer only, use remainder in step 2) n Step 2: m = n + remainder from step 1

Rule of Thirteen o Example: The formula for a hydrocarbon with M+ =106 can be found: n Step 1: n = 106/13 = 8 (R = 2) n Step 2: m = 8 + 2 = 10 n Formula: C 8 H 10
Rule of Thirteen o If a heteroatom is present, n Subtract the mass of each heteroatom from the MW n Calculate the formula for the corresponding hydrocarbon n Add the heteroatoms to the formula
Rule of Thirteen Example: A compound with a molecular ion peak at m/z = 102 has a strong peak at 1739 cm-1 in its IR spectrum. Determine its molecular formula.

GC-Mass Spec: Experiment 23 o Mass Spec can be combined with gas chromatography to analyze mixtures of compounds. n GC separates the components of the mixture. n Each component is analyzed by the Mass Spectrometer.

GC-Mass Spec: Experiment 23 o Assignment: n Observe the GC-mass spec experiment o Record experimental conditions n Analyze the mass spectrum of each component of your mixture: o Parent ion peak? o Heteroatoms apparent from spectrum? o A minimum of 1 or two significant fragments and their structures
GC-Mass Spec: Experiment 23 o Assignment (cont. ): n Using the Mass Spec data, retention times, and boiling points, identify the components of your mixture. n Write three paragraphs (one per compound) summarizing and interpreting all data. See your data sheet for more details.
- Slides: 54