Units and Measurement Physics Mrs Coyle International Space













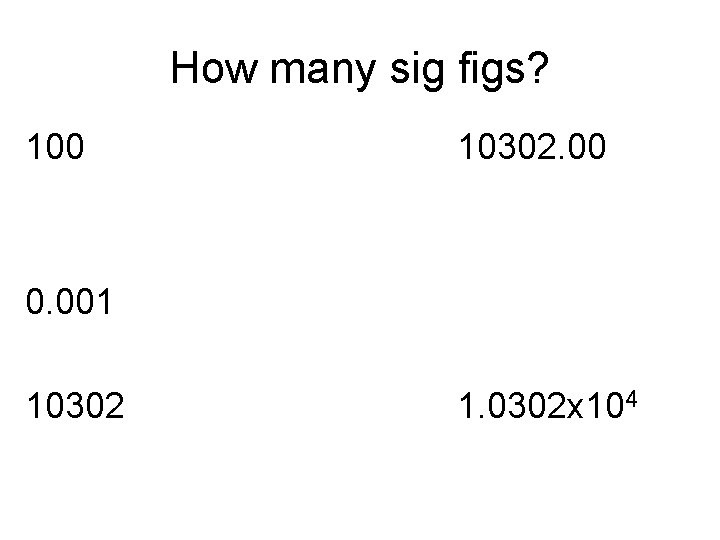



- Slides: 29

Units and Measurement Physics Mrs. Coyle International Space Station http: //apod. nasa. gov/apod/image/0706/iss_sts 117_big. jpg

It All Starts with a Ruler!!!

Math and Units • Math- the language of Physics • SI Units – International System – MKS • Meter m • Mass kg • Time s • National Bureau of Standards • Prefixes

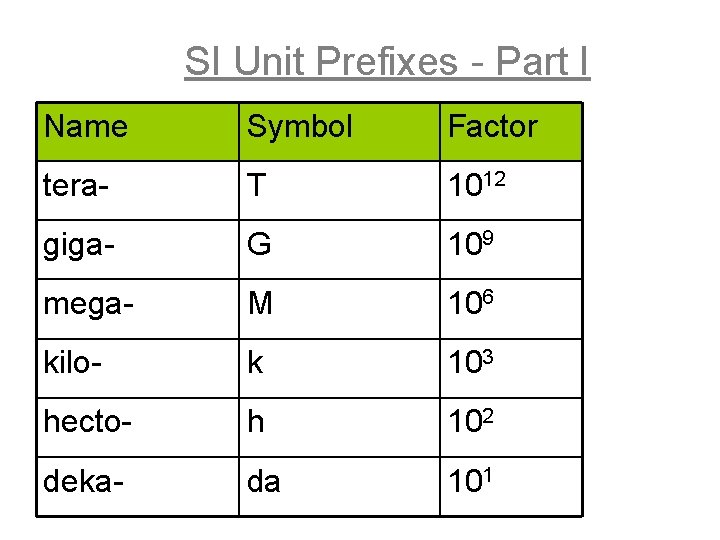
SI Unit Prefixes - Part I Name Symbol Factor tera- T 1012 giga- G 109 mega- M 106 kilo- k 103 hecto- h 102 deka- da 101

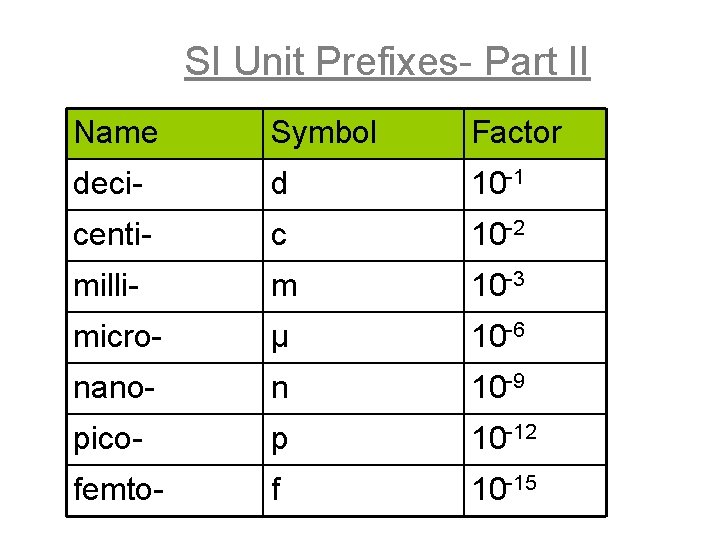
SI Unit Prefixes- Part II Name Symbol Factor deci- d 10 -1 centi- c 10 -2 milli- m 10 -3 micro- μ 10 -6 nano- n 10 -9 pico- p 10 -12 femto- f 10 -15

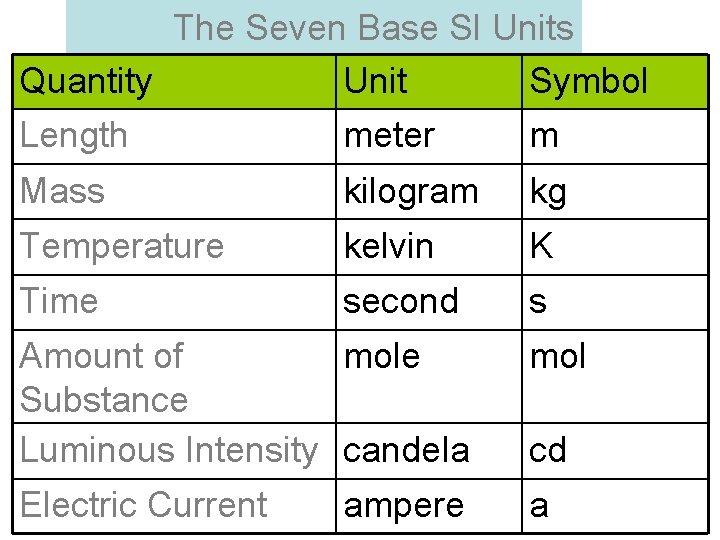
The Seven Base SI Units Quantity Unit Symbol Length meter m Mass kilogram kg Temperature kelvin K Time second s Amount of mole Substance Luminous Intensity candela mol Electric Current a ampere cd

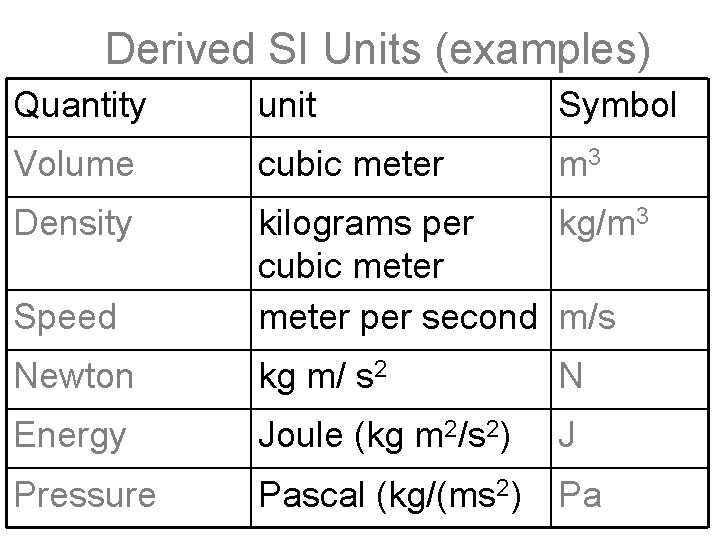
Derived SI Units (examples) Quantity unit Symbol Volume cubic meter m 3 Density Speed kilograms per kg/m 3 cubic meter per second m/s Newton kg m/ s 2 N Energy Joule (kg m 2/s 2) J Pressure Pascal (kg/(ms 2) Pa

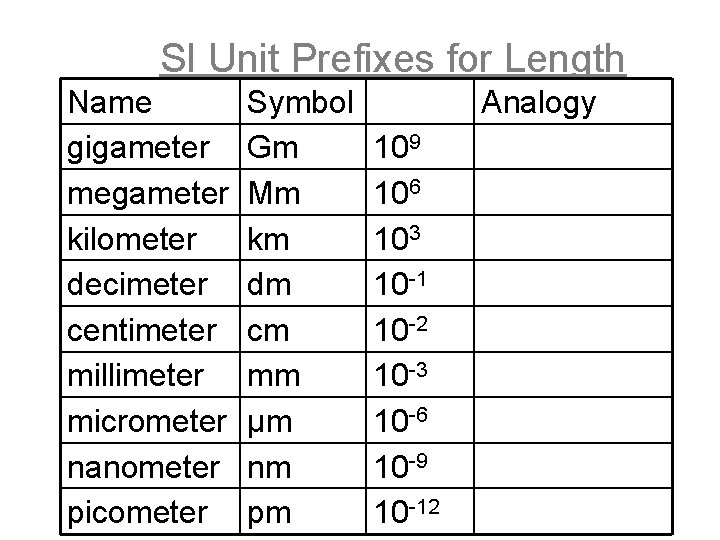
SI Unit Prefixes for Length Name gigameter megameter kilometer decimeter centimeter millimeter micrometer nanometer picometer Symbol Gm Mm km dm cm mm μm nm pm Analogy 109 106 103 10 -1 10 -2 10 -3 10 -6 10 -9 10 -12

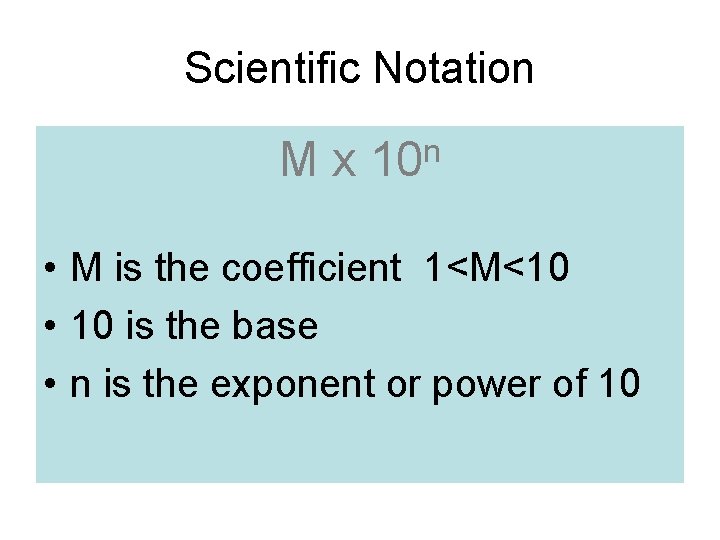
Scientific Notation Mx n 10 • M is the coefficient 1<M<10 • 10 is the base • n is the exponent or power of 10

Other Examples: • 5. 45 E+6 • 5. 45 x 10^6 or

Numbers less than 1 will have a negative exponent. A millionth of a second is: 0. 000001 sec 1. 0 E-6 1 x 10 -6 1. 0^-6

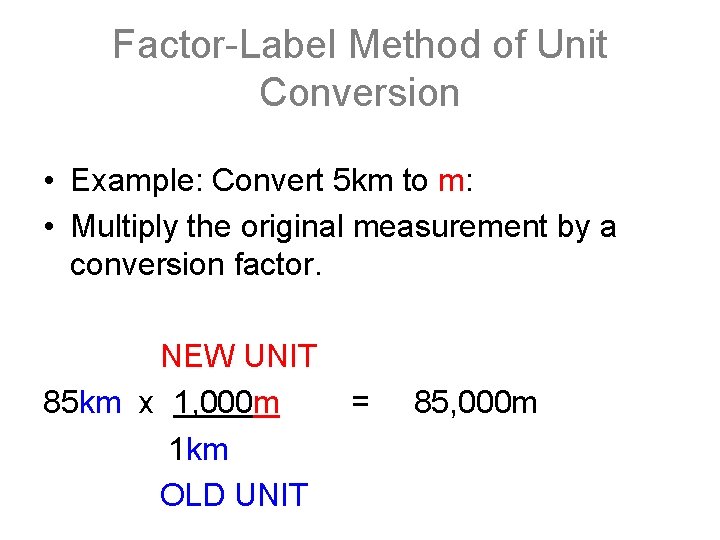
Factor-Label Method of Unit Conversion • Example: Convert 5 km to m: • Multiply the original measurement by a conversion factor. NEW UNIT 85 km x 1, 000 m 1 km OLD UNIT = 85, 000 m

Factor-Label Method of Unit Conversion: Example • Example: Convert 789 m to km: 789 m x 1 km =0. 789 km= 7. 89 x 10 -1 km 1000 m

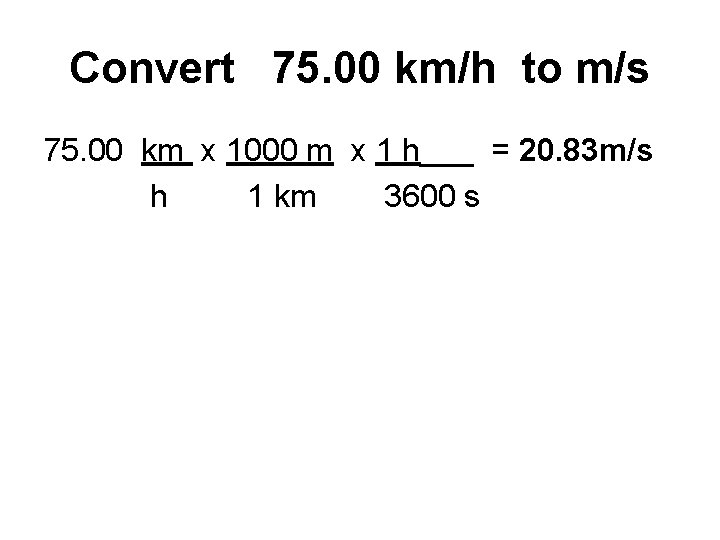
Convert 75. 00 km/h to m/s 75. 00 km x 1000 m x 1 h___ = 20. 83 m/s h 1 km 3600 s

Limits of Measurement • Accuracy and Precision

• Accuracy - a measure of how close a measurement is to the true value of the quantity being measured.

Example: Accuracy • Who is more accurate when measuring a book that has a true length of 17. 0 cm? Susan: 17. 0 cm, 16. 0 cm, 18. 0 cm, 15. 0 cm Amy: 15. 5 cm, 15. 0 cm, 15. 2 cm, 15. 3 cm

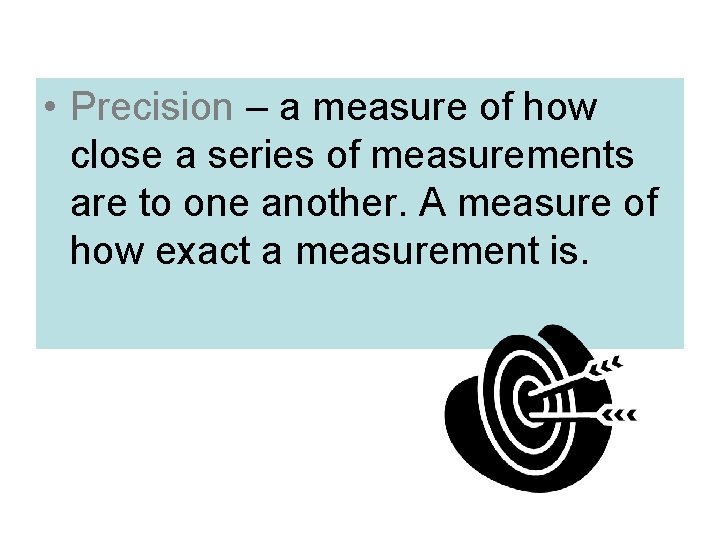
• Precision – a measure of how close a series of measurements are to one another. A measure of how exact a measurement is.

Example: Precision Who is more precise when measuring the same 17. 0 cm book? Susan: 17. 0 cm, 16. 0 cm, 18. 0 cm, 15. 0 cm Amy: 15. 5 cm, 15. 0 cm, 15. 2 cm, 15. 3 cm


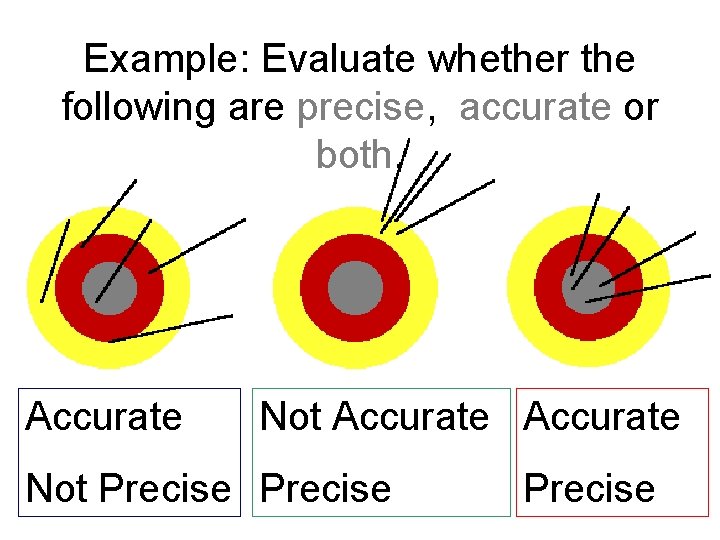
Example: Evaluate whether the following are precise, accurate or both. Accurate Not Precise

Significant Figures • The significant figures in a measurement include all of the digits that are known, plus one last digit that is estimated.

Centimeters and Millimeters

Finding the Number of Sig Figs: • When the decimal is present, start counting from the left. • When the decimal is absent, start counting from the right. • Zeroes encountered before a non zero digit do not count.
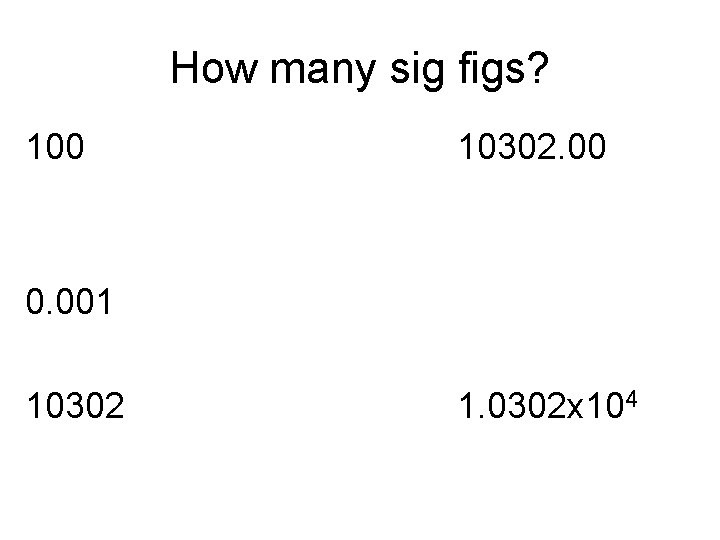
How many sig figs? 100 10302. 00 0. 001 10302 1. 0302 x 104

Sig Figs in Addition/Subtraction Express the result with the same number of decimal places as the number in the operation with the least decimal places. Ex: 2. 33 cm + 3. 0 cm 5. 3 cm (Result is rounded to one decimal place)

Sig Figs in Multiplication/Division • Express the answer with the same sig figs as the factor with the least sig figs. • Ex: 3. 22 cm x 2. 0 cm 6. 4 cm 2 (Result is rounded to two sig figs)

Counting Numbers • Counting numbers have infinite sig figs. • Ex: 3 apples

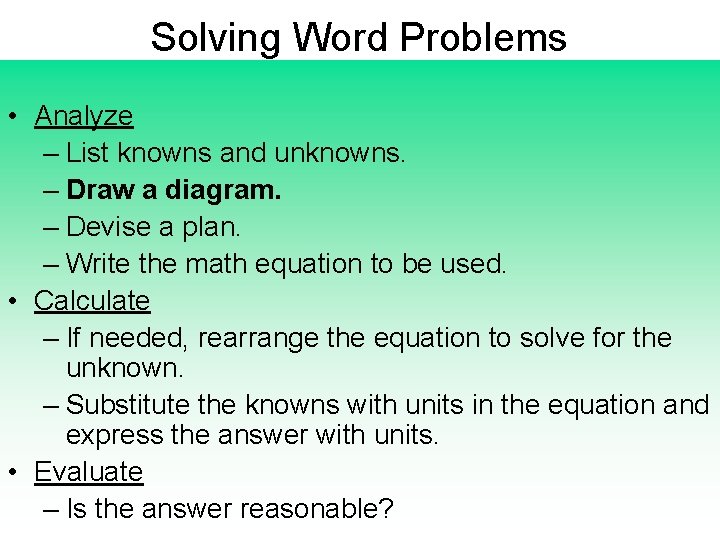
Solving Word Problems • Analyze – List knowns and unknowns. – Draw a diagram. – Devise a plan. – Write the math equation to be used. • Calculate – If needed, rearrange the equation to solve for the unknown. – Substitute the knowns with units in the equation and express the answer with units. • Evaluate – Is the answer reasonable?
 Physical quantities
Physical quantities Units of measurement in physics
Units of measurement in physics They are mrs garcia and mrs castro
They are mrs garcia and mrs castro They are mrs garcia and mrs castro
They are mrs garcia and mrs castro Mrs. darling was ___________ of mrs. s.
Mrs. darling was ___________ of mrs. s. Mussd
Mussd Coyle and castello method
Coyle and castello method Coyle health and wellbeing
Coyle health and wellbeing Mrs gallon
Mrs gallon Units of momentum physics
Units of momentum physics 3 units of linear measurements in metric system
3 units of linear measurements in metric system Customary units of measurement
Customary units of measurement Typical room height metric unit
Typical room height metric unit Customary units of measurement
Customary units of measurement Deci centi mili
Deci centi mili Customary units of measurement
Customary units of measurement Metric system table
Metric system table Units of length smallest to largest
Units of length smallest to largest Which countries use imperial system
Which countries use imperial system Unit conversion staircase
Unit conversion staircase Converting metric lengths
Converting metric lengths Coyle
Coyle Coyle method
Coyle method Caitlin coyle
Caitlin coyle Do coyle
Do coyle Coyle bernard
Coyle bernard Coyle
Coyle Coyle electric
Coyle electric Aaron coyle
Aaron coyle Do coyle
Do coyle