Limiting Reagent Chemistry Mrs Coyle http www kitmondo





- Slides: 8

Limiting Reagent Chemistry Mrs. Coyle http: //www. kitmondo. com/images%5 Clisting%5 CDe%20 Dietrich%20 GL-750%20 Glass-Lined%20 Jacketed%20 Reactor-%20 MT-1%20(Pic 1). JPG

¡ What happens in a chemical reaction, if there is an insufficient amount of one reactant? http: //ossfabricators. com/photos/reactor 2. jpg

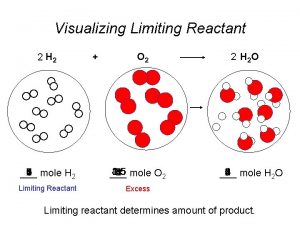
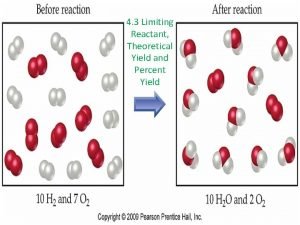
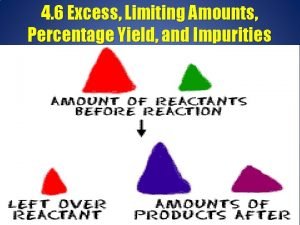
¡ ¡ Limiting Reagent: the reagent that is completely used up in a chemical reaction. Excess Reagent: reagent not completely used up in a chemical reaction.

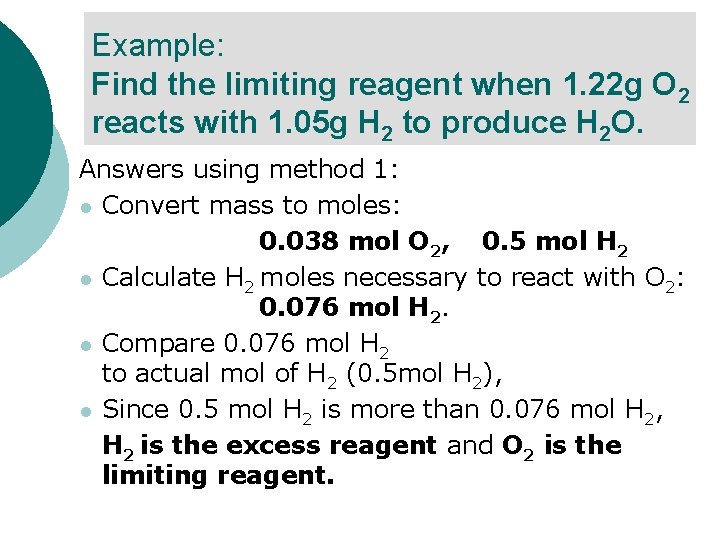
Example: Find the limiting reagent when 1. 22 g O 2 reacts with 1. 05 g H 2 to produce H 2 O. ¡ ¡ There are two solution methods you could use. In both methods, the first step is to convert the mass to moles.

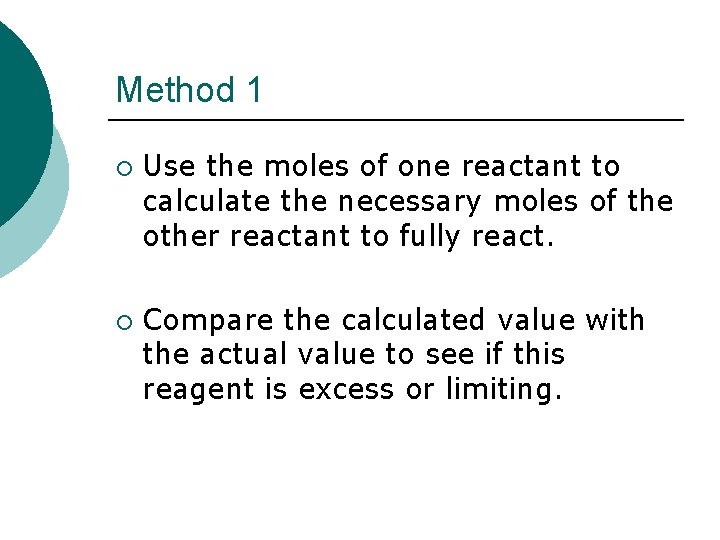
Method 1 ¡ ¡ Use the moles of one reactant to calculate the necessary moles of the other reactant to fully react. Compare the calculated value with the actual value to see if this reagent is excess or limiting.

Example: Find the limiting reagent when 1. 22 g O 2 reacts with 1. 05 g H 2 to produce H 2 O. Answers using method 1: l Convert mass to moles: 0. 038 mol O 2, 0. 5 mol H 2 l Calculate H 2 moles necessary to react with O 2: 0. 076 mol H 2. l Compare 0. 076 mol H 2 to actual mol of H 2 (0. 5 mol H 2), l Since 0. 5 mol H 2 is more than 0. 076 mol H 2, H 2 is the excess reagent and O 2 is the limiting reagent.

Method 2 ¡ ¡ ¡ Use the moles of each of the reactant to calculate one of the products. The reagent that gave the smaller calculated value of product is the limiting reagent. The actual value of the amount of product is the smaller of the calculated values.

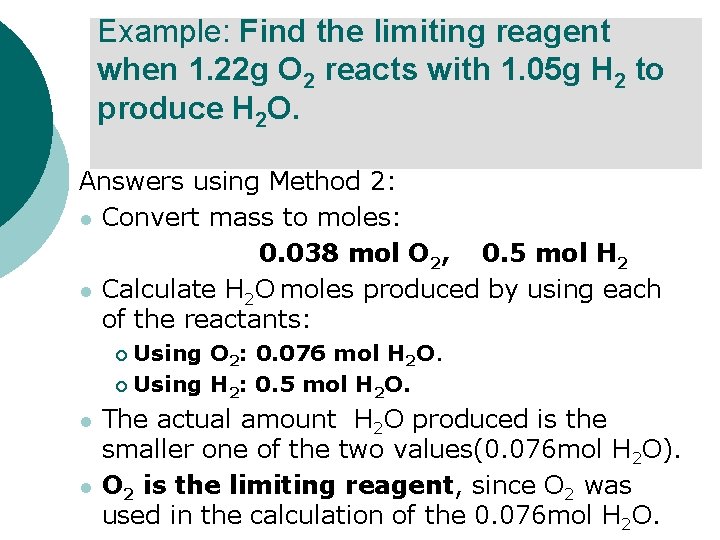
Example: Find the limiting reagent when 1. 22 g O 2 reacts with 1. 05 g H 2 to produce H 2 O. Answers using Method 2: l Convert mass to moles: 0. 038 mol O 2, 0. 5 mol H 2 l Calculate H 2 O moles produced by using each of the reactants: Using O 2: 0. 076 mol H 2 O. ¡ Using H 2: 0. 5 mol H 2 O. ¡ l l The actual amount H 2 O produced is the smaller one of the two values(0. 076 mol H 2 O). O 2 is the limiting reagent, since O 2 was used in the calculation of the 0. 076 mol H 2 O.