LIMITING REACTANT LIMITING REACTANT Limiting Reagent The reactant




- Slides: 8

LIMITING REACTANT

LIMITING REACTANT • Limiting Reagent • The reactant that is totally used up during the chemical reaction • Limits the amount of product produced

EXCESS REACTANT • Excess Reagent • The reactant present in a quantity that is greater than what is needed to react with the limiting reactant • Remains after the limiting reactant has been used up

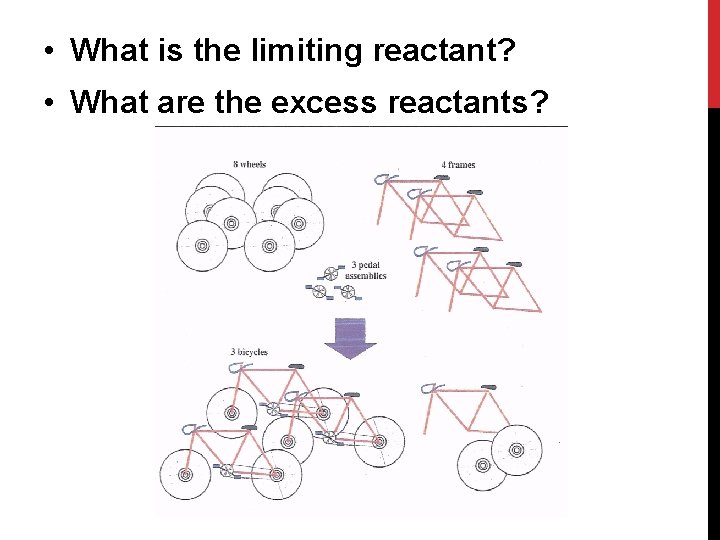
• What is the limiting reactant? • What are the excess reactants?

STEPS 1. Balance the equation 2. Identify the givens (there are 2!) 3. Identify what you want to find 4. Use stoichiometry to solve 5. The reactant with a smaller amount of product produced is the limiting reactant 6. The other reactant is in excess

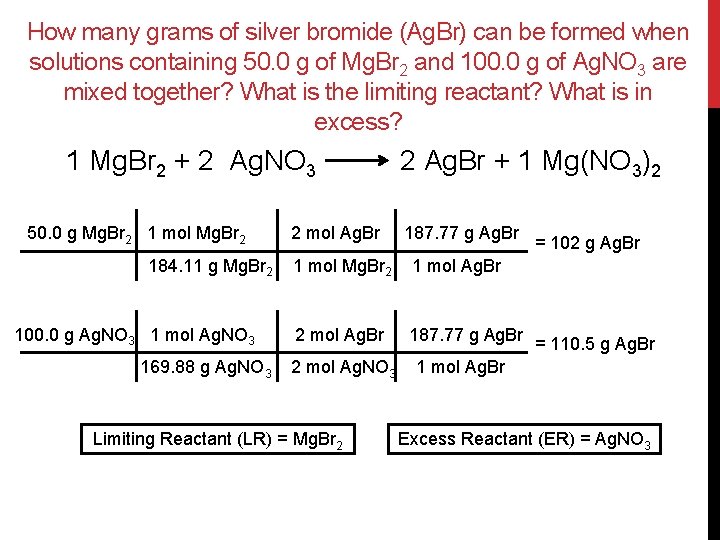
How many grams of silver bromide (Ag. Br) can be formed when solutions containing 50. 0 g of Mg. Br 2 and 100. 0 g of Ag. NO 3 are mixed together? What is the limiting reactant? What is in excess? 1 Mg. Br 2 + 2 Ag. NO 3 50. 0 g Mg. Br 2 1 mol Mg. Br 2 184. 11 g Mg. Br 2 100. 0 g Ag. NO 3 1 mol Ag. NO 3 169. 88 g Ag. NO 3 2 mol Ag. Br 2 Ag. Br + 1 Mg(NO 3)2 187. 77 g Ag. Br 1 mol Mg. Br 2 1 mol Ag. Br 2 mol Ag. Br 187. 77 g Ag. Br 2 mol Ag. NO 3 Limiting Reactant (LR) = Mg. Br 2 = 102 g Ag. Br = 110. 5 g Ag. Br 1 mol Ag. Br Excess Reactant (ER) = Ag. NO 3

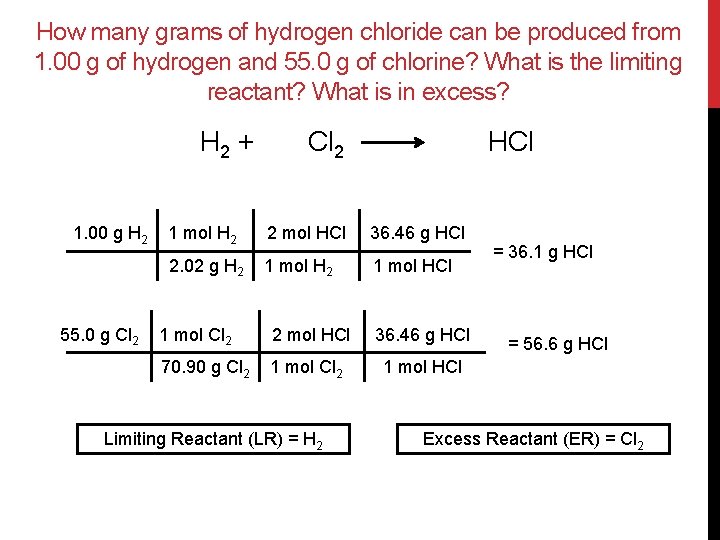
How many grams of hydrogen chloride can be produced from 1. 00 g of hydrogen and 55. 0 g of chlorine? What is the limiting reactant? What is in excess? H 2 + 1. 00 g H 2 55. 0 g Cl 2 HCl 1 mol H 2 2 mol HCl 36. 46 g HCl 2. 02 g H 2 1 mol HCl 1 mol Cl 2 2 mol HCl 36. 46 g HCl 70. 90 g Cl 2 1 mol HCl Limiting Reactant (LR) = H 2 = 36. 1 g HCl = 56. 6 g HCl Excess Reactant (ER) = Cl 2

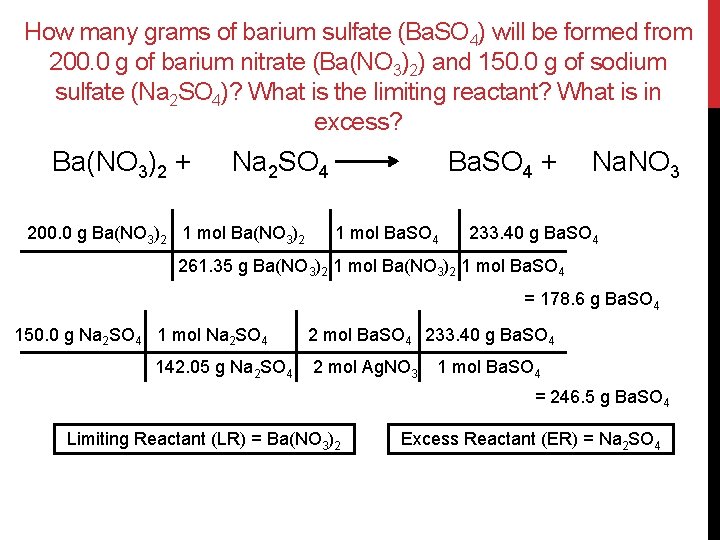
How many grams of barium sulfate (Ba. SO 4) will be formed from 200. 0 g of barium nitrate (Ba(NO 3)2) and 150. 0 g of sodium sulfate (Na 2 SO 4)? What is the limiting reactant? What is in excess? Ba(NO 3)2 + Na 2 SO 4 200. 0 g Ba(NO 3)2 1 mol Ba(NO 3)2 Ba. SO 4 + 1 mol Ba. SO 4 Na. NO 3 233. 40 g Ba. SO 4 261. 35 g Ba(NO 3)2 1 mol Ba. SO 4 = 178. 6 g Ba. SO 4 150. 0 g Na 2 SO 4 1 mol Na 2 SO 4 142. 05 g Na 2 SO 4 2 mol Ba. SO 4 233. 40 g Ba. SO 4 2 mol Ag. NO 3 1 mol Ba. SO 4 = 246. 5 g Ba. SO 4 Limiting Reactant (LR) = Ba(NO 3)2 Excess Reactant (ER) = Na 2 SO 4