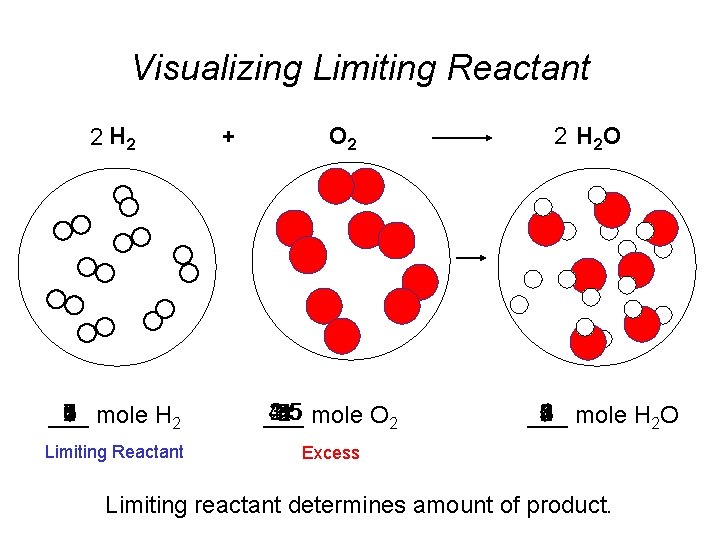
Visualizing Limiting Reactant 2 H 2 O 2




- Slides: 25

Visualizing Limiting Reactant 2 H 2 + O 2 7 0 8 3 4 2 6 5 mole H 2 1 ___ 2. 5 1. 5 4. 5 3. 5 1 5 3 mole O 2 2 4 ___ Limiting Reactant Excess 2 H 2 O 1 5 8 0 4 6 2 3 mole H 2 O 7 ___ Limiting reactant determines amount of product.

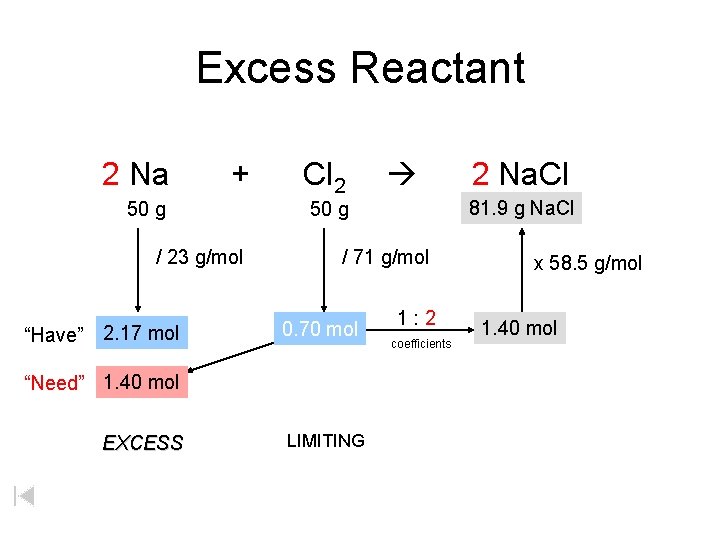
Excess Reactant 2 Na + 50 g / 23 g/mol “Have” 2. 17 mol Cl 2 / 71 g/mol 0. 70 mol LIMITING 2 Na. Cl 81. 9 xg g. Na. Cl 50 g “Need” 1. 40 mol EXCESS 1: 2 coefficients x 58. 5 g/mol 1. 40 mol

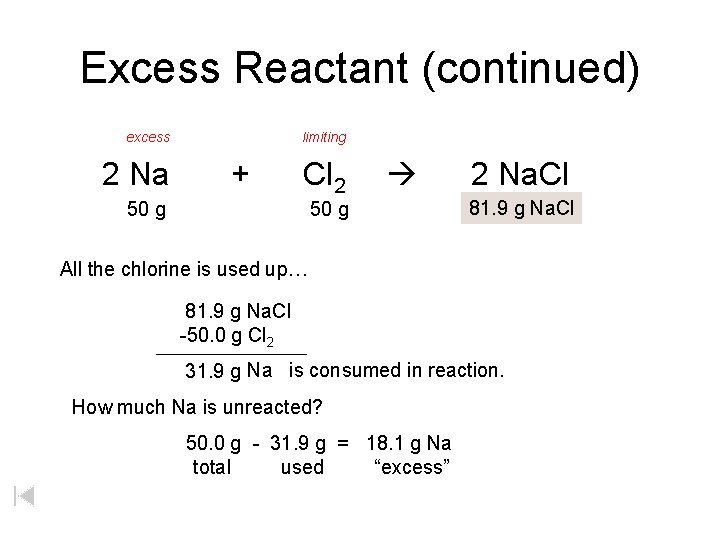
Excess Reactant (continued) excess 2 Na limiting + Cl 2 50 g 2 Na. Cl 81. 9 xg g. Na. Cl All the chlorine is used up… 81. 9 g Na. Cl -50. 0 g Cl 2 31. 9 g Na is consumed in reaction. How much Na is unreacted? 50. 0 g - 31. 9 g = 18. 1 g Na total used “excess”

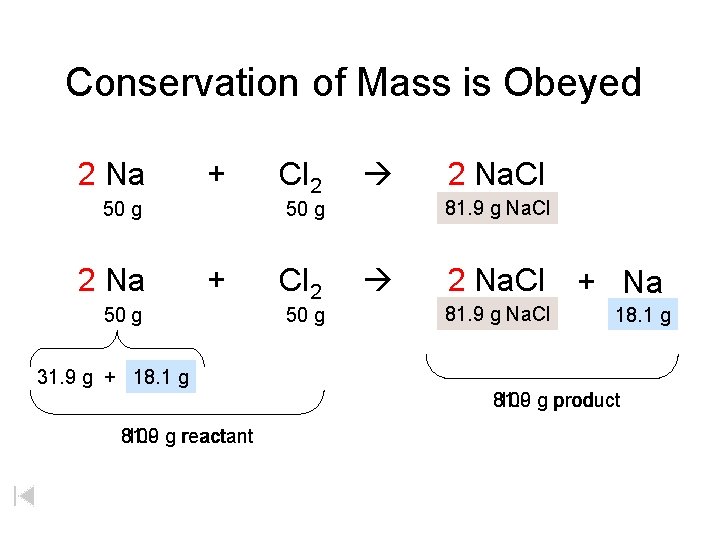
Conservation of Mass is Obeyed 2 Na + 50 g 2 Na Cl 2 81. 9 xg g. Na. Cl 50 g + 50 g Cl 2 50 g 2 Na. Cl + Na 81. 9 xgg. Na. Cl 18. 1 g 31. 9 g + 18. 1 g 100 g product 81. 9 100 g reactant 81. 9

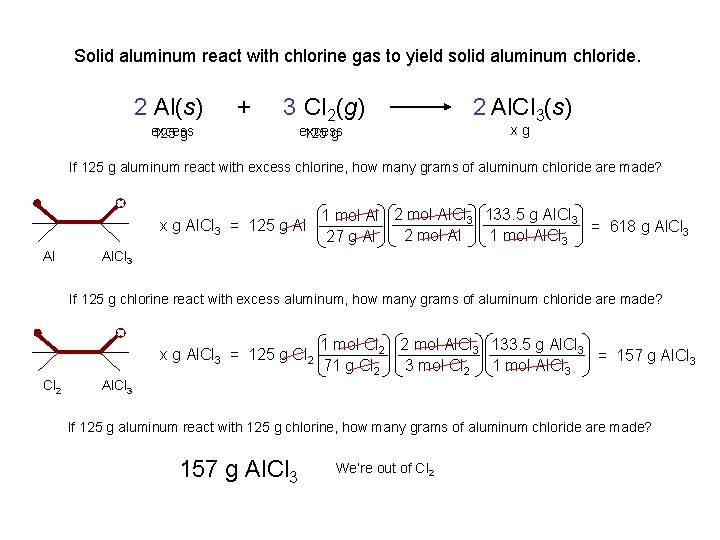
Solid aluminum react with chlorine gas to yield solid aluminum chloride. 2 Al(s) excess 125 g + 3 Cl 2(g) excess 125 g 2 Al. Cl 3(s) xg If 125 g aluminum react with excess chlorine, how many grams of aluminum chloride are made? x g Al. Cl 3 = 125 g Al Al 1 mol Al 2 mol Al. Cl 3 133. 5 g Al. Cl 3 = 618 g Al. Cl 3 2 mol Al 1 mol Al. Cl 3 27 g Al Al. Cl 3 If 125 g chlorine react with excess aluminum, how many grams of aluminum chloride are made? x g Al. Cl 3 = 125 g Cl 2 1 mol Cl 2 2 mol Al. Cl 3 133. 5 g Al. Cl 3 = 157 g Al. Cl 3 3 mol Cl 2 1 mol Al. Cl 3 71 g Cl 2 Al. Cl 3 If 125 g aluminum react with 125 g chlorine, how many grams of aluminum chloride are made? 157 g Al. Cl 3 We’re out of Cl 2

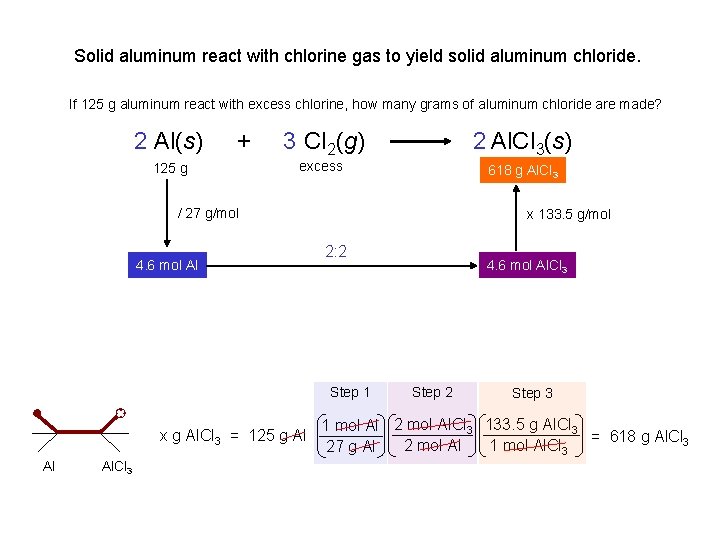
Solid aluminum react with chlorine gas to yield solid aluminum chloride. If 125 g aluminum react with excess chlorine, how many grams of aluminum chloride are made? 2 Al(s) + 125 g 3 Cl 2(g) 2 Al. Cl 3(s) 618 xgg. Al. Cl 3 excess / 27 g/mol 4. 6 mol Al x 133. 5 g/mol 2: 2 Step 1 x g Al. Cl 3 = 125 g Al Al Al. Cl 3 4. 6 mol Al. Cl 3 Step 2 Step 3 1 mol Al 2 mol Al. Cl 3 133. 5 g Al. Cl 3 = 618 g Al. Cl 3 2 mol Al 1 mol Al. Cl 3 27 g Al

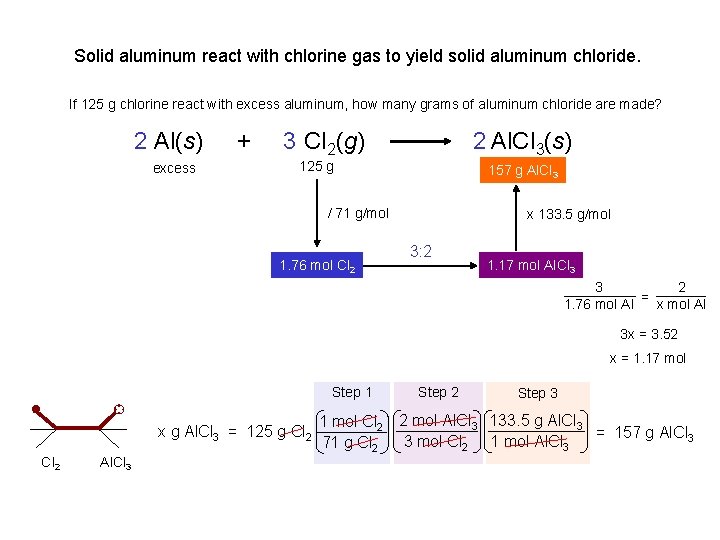
Solid aluminum react with chlorine gas to yield solid aluminum chloride. If 125 g chlorine react with excess aluminum, how many grams of aluminum chloride are made? 2 Al(s) excess + 3 Cl 2(g) 2 Al. Cl 3(s) 157 xgg. Al. Cl 3 125 g / 71 g/mol 1. 76 mol Cl 2 x 133. 5 g/mol 3: 2 1. 17 mol Al. Cl 3 3 2 = 1. 76 mol Al x mol Al 3 x = 3. 52 x = 1. 17 mol Step 1 x g Al. Cl 3 = 125 g Cl 2 Al. Cl 3 Step 2 Step 3 1 mol Cl 2 2 mol Al. Cl 3 133. 5 g Al. Cl 3 = 157 g Al. Cl 3 3 mol Cl 2 1 mol Al. Cl 3 71 g Cl 2

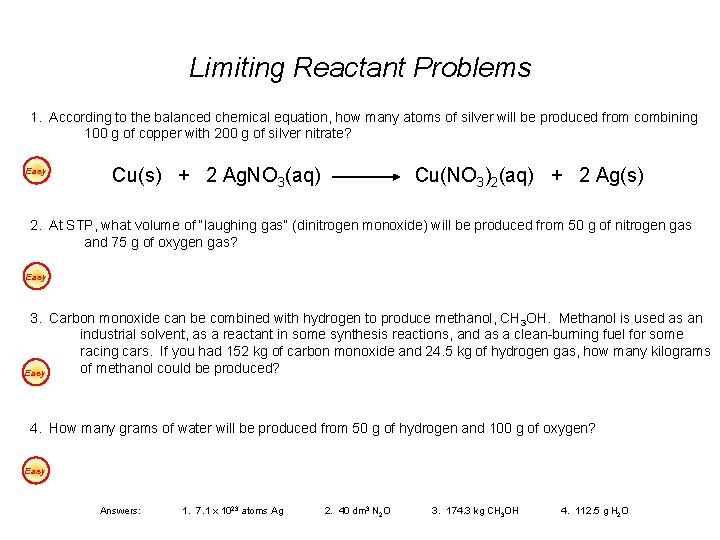
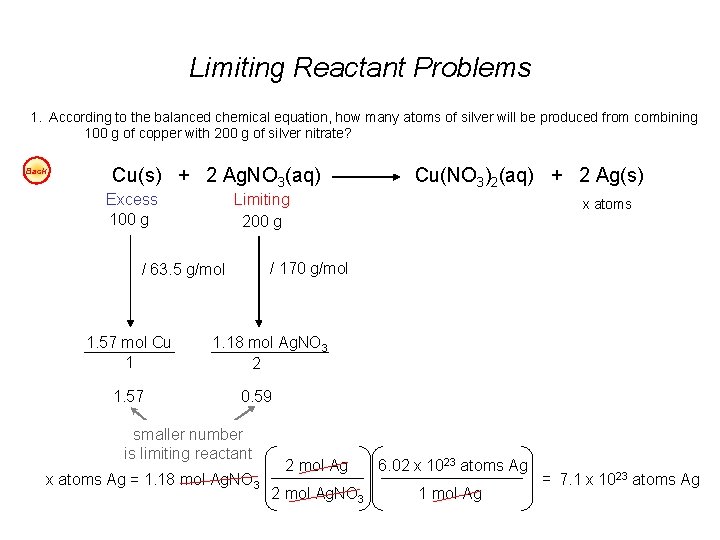
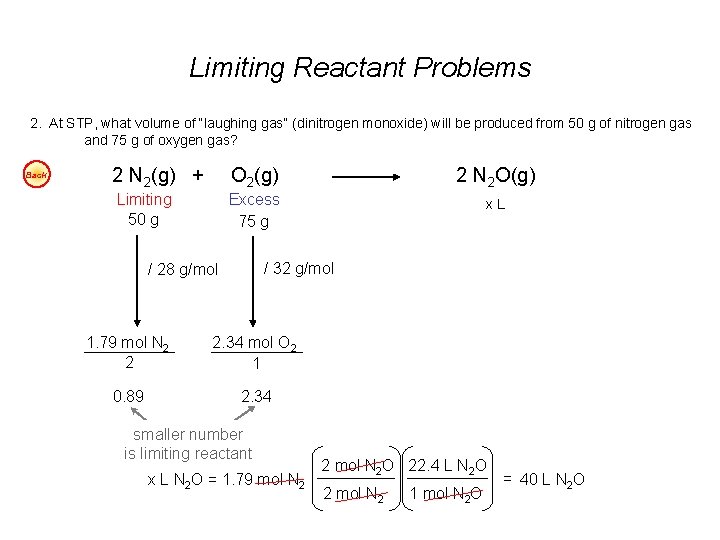
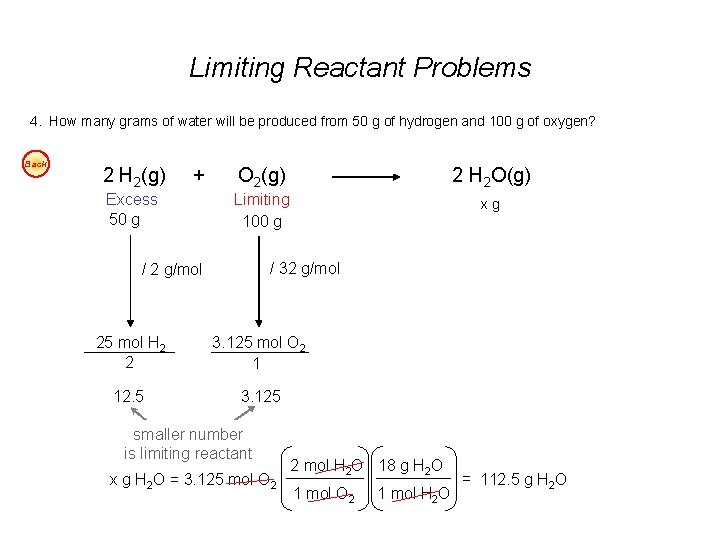
Limiting Reactant Problems 1. According to the balanced chemical equation, how many atoms of silver will be produced from combining 100 g of copper with 200 g of silver nitrate? Easy Cu(s) + 2 Ag. NO 3(aq) Cu(NO 3)2(aq) + 2 Ag(s) 2. At STP, what volume of “laughing gas” (dinitrogen monoxide) will be produced from 50 g of nitrogen gas and 75 g of oxygen gas? Easy 3. Carbon monoxide can be combined with hydrogen to produce methanol, CH 3 OH. Methanol is used as an industrial solvent, as a reactant in some synthesis reactions, and as a clean-burning fuel for some racing cars. If you had 152 kg of carbon monoxide and 24. 5 kg of hydrogen gas, how many kilograms of methanol could be produced? Easy 4. How many grams of water will be produced from 50 g of hydrogen and 100 g of oxygen? Easy Answers: 1. 7. 1 x 1023 atoms Ag 2. 40 dm 3 N 2 O 3. 174. 3 kg CH 3 OH 4. 112. 5 g H 2 O

Limiting Reactant Problems 1. According to the balanced chemical equation, how many atoms of silver will be produced from combining 100 g of copper with 200 g of silver nitrate? Back Cu(s) + 2 Ag. NO 3(aq) Excess 100 g Limiting 200 g 1. 57 x atoms / 170 g/mol / 63. 5 g/mol 1. 57 mol Cu 1 Cu(NO 3)2(aq) + 2 Ag(s) 1. 18 mol Ag. NO 3 2 0. 59 smaller number is limiting reactant x atoms Ag = 1. 18 mol Ag. NO 3 2 mol Ag 6. 02 x 1023 atoms Ag 2 mol Ag. NO 3 1 mol Ag = 7. 1 x 1023 atoms Ag

Limiting Reactant Problems 2. At STP, what volume of “laughing gas” (dinitrogen monoxide) will be produced from 50 g of nitrogen gas and 75 g of oxygen gas? Back 2 N 2(g) + O 2(g) 2 N 2 O(g) Limiting 50 g Excess 75 g x. L / 32 g/mol / 28 g/mol 1. 79 mol N 2 2 2. 34 mol O 2 1 0. 89 2. 34 smaller number is limiting reactant x L N 2 O = 1. 79 mol N 2 2 mol N 2 O 22. 4 L N 2 O 2 mol N 2 1 mol N 2 O = 40 L N 2 O

Limiting Reactant Problems 3. Carbon monoxide can be combined with hydrogen to produce methanol, CH 3 OH. Methanol is used as an industrial solvent, as a reactant in some synthesis reactions, and as a clean-burning fuel for some racing cars. If you had 152 kg of carbon monoxide and 24. 5 kg of hydrogen gas, how many kilograms of methanol could be produced? Back CO (g) + 2 H 2(g) Limiting 152. 5 g Excess 24. 5 g CH 3 OH (g) x g kg 174. 3 / 2 g/mol / 28 g/mol 5. 45 mol CO 1 12. 25 mol H 2 2 5. 45 6. 125 smaller number is limiting reactant x g CH 3 OH = 5. 45 mol CO Work the entire problem with the mass in grams. At the end, change answer to units of kilograms. 1 mol CH 3 OH 1 mol CO 32 g CH 3 OH 1 mol CH 3 OH = 174. 3 g CH 3 OH

Limiting Reactant Problems 4. How many grams of water will be produced from 50 g of hydrogen and 100 g of oxygen? Back 2 H 2(g) + Excess 50 g O 2(g) 2 H 2 O(g) Limiting 100 g xg / 32 g/mol / 2 g/mol 25 mol H 2 2 3. 125 mol O 2 1 12. 5 3. 125 smaller number is limiting reactant x g H 2 O = 3. 125 mol O 2 2 mol H 2 O 18 g H 2 O 1 mol O 2 1 mol H 2 O = 112. 5 g H 2 O

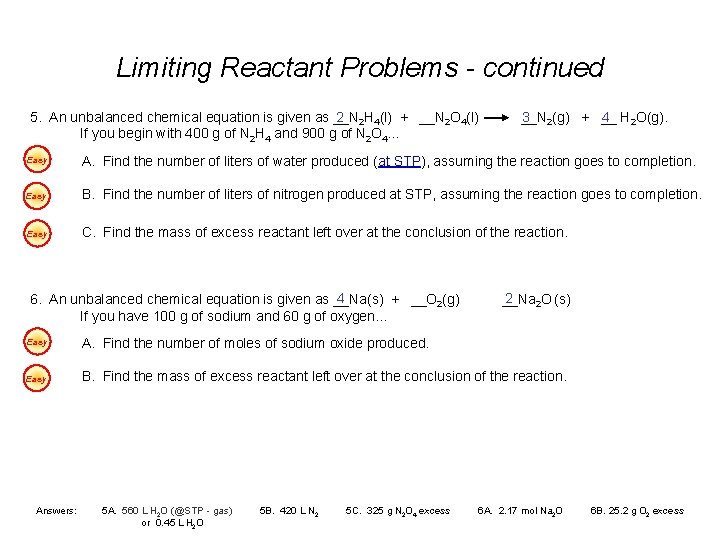
Limiting Reactant Problems - continued 2 2 H 4(l) + __N 2 O 4(l) 5. An unbalanced chemical equation is given as __N If you begin with 400 g of N 2 H 4 and 900 g of N 2 O 4… 3 2(g) + __ 4 H 2 O(g). __N Easy A. Find the number of liters of water produced (at STP), assuming the reaction goes to completion. Easy B. Find the number of liters of nitrogen produced at STP, assuming the reaction goes to completion. Easy C. Find the mass of excess reactant left over at the conclusion of the reaction. 4 6. An unbalanced chemical equation is given as __Na(s) + __O 2(g) If you have 100 g of sodium and 60 g of oxygen… 2 __Na 2 O (s) Easy A. Find the number of moles of sodium oxide produced. Easy B. Find the mass of excess reactant left over at the conclusion of the reaction. Answers: 5 A. 560 L H 2 O (@STP - gas) or 0. 45 L H 2 O 5 B. 420 L N 2 5 C. 325 g N 2 O 4 excess 6 A. 2. 17 mol Na 2 O 6 B. 25. 2 g O 2 excess

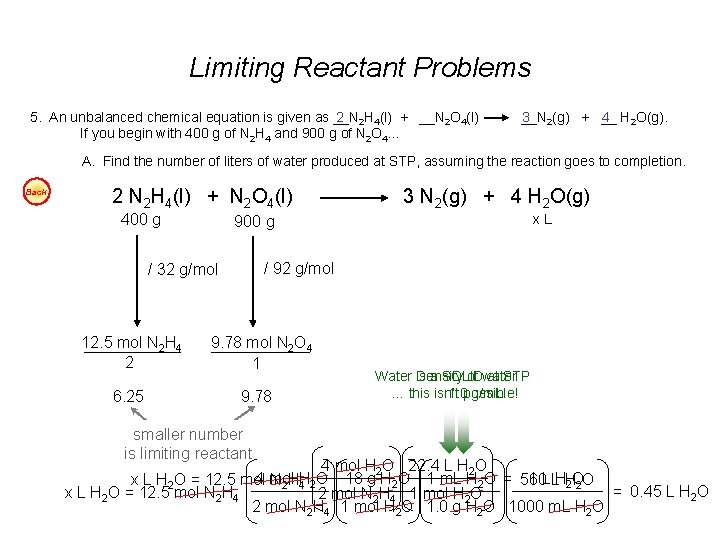
Limiting Reactant Problems 5. An unbalanced chemical equation is given as __N 2 2 H 4(l) + __N 2 O 4(l) If you begin with 400 g of N 2 H 4 and 900 g of N 2 O 4… __N 3 2(g) + __ 4 H 2 O(g). A. Find the number of liters of water produced at STP, assuming the reaction goes to completion. Back 2 N 2 H 4(l) + N 2 O 4(l) 400 g 6. 25 x. L 900 g / 92 g/mol / 32 g/mol 12. 5 mol N 2 H 4 2 3 N 2(g) + 4 H 2 O(g) 9. 78 mol N 2 O 4 1 9. 78 smaller number is limiting reactant Water is Density a SOLID of water at STP … this is isn’t 1. 0 possible! g/m. L 4 mol H 2 O 22. 4 L H 2 O 4 mol H O 18 g H 2 O 1 m. L H 2 O = 560 1 L LHH x L H 2 O = 12. 5 mol N 2 H 4 2 2 O 2 O = 0. 45 L H 2 O x L H 2 O = 12. 5 mol N 2 H 4 2 mol N 2 H 4 1 mol H 2 O 1. 0 g H 2 O 1000 m. L H 2 O

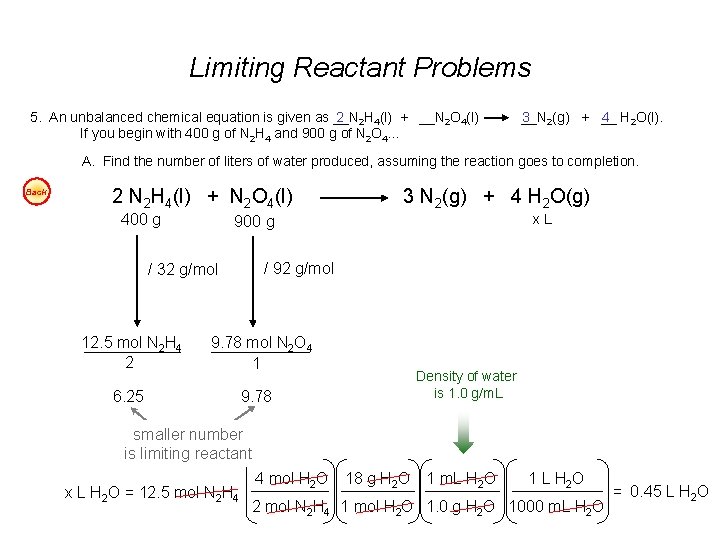
Limiting Reactant Problems 5. An unbalanced chemical equation is given as __N 2 2 H 4(l) + __N 2 O 4(l) If you begin with 400 g of N 2 H 4 and 900 g of N 2 O 4… __N 3 2(g) + __ 4 H 2 O(l). A. Find the number of liters of water produced, assuming the reaction goes to completion. Back 2 N 2 H 4(l) + N 2 O 4(l) 400 g x. L 900 g / 92 g/mol / 32 g/mol 12. 5 mol N 2 H 4 2 3 N 2(g) + 4 H 2 O(g) 9. 78 mol N 2 O 4 1 6. 25 Density of water is 1. 0 g/m. L 9. 78 smaller number is limiting reactant x L H 2 O = 12. 5 mol N 2 H 4 4 mol H 2 O 18 g H 2 O 1 m. L H 2 O 1 L H 2 O 2 mol N 2 H 4 1 mol H 2 O 1. 0 g H 2 O 1000 m. L H 2 O = 0. 45 L H 2 O

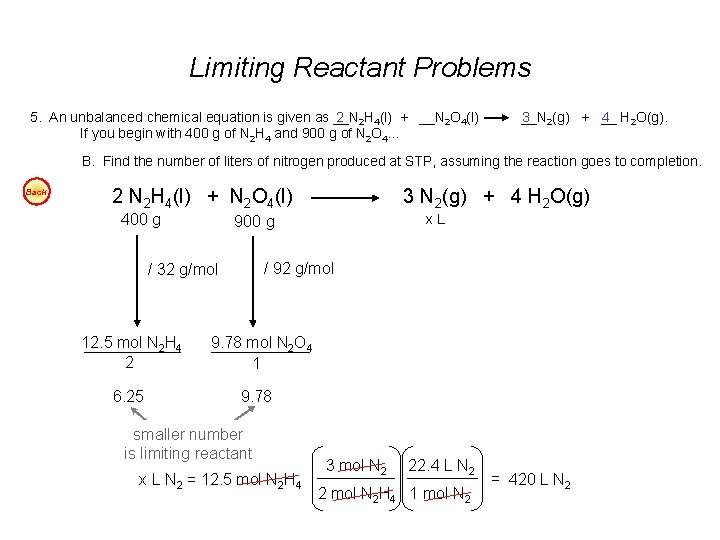
Limiting Reactant Problems 5. An unbalanced chemical equation is given as __N 2 2 H 4(l) + __N 2 O 4(l) If you begin with 400 g of N 2 H 4 and 900 g of N 2 O 4… __N 3 2(g) + __ 4 H 2 O(g). B. Find the number of liters of nitrogen produced at STP, assuming the reaction goes to completion. Back 2 N 2 H 4(l) + N 2 O 4(l) 400 g 6. 25 x. L 900 g / 92 g/mol / 32 g/mol 12. 5 mol N 2 H 4 2 3 N 2(g) + 4 H 2 O(g) 9. 78 mol N 2 O 4 1 9. 78 smaller number is limiting reactant x L N 2 = 12. 5 mol N 2 H 4 3 mol N 2 22. 4 L N 2 2 mol N 2 H 4 1 mol N 2 = 420 L N 2

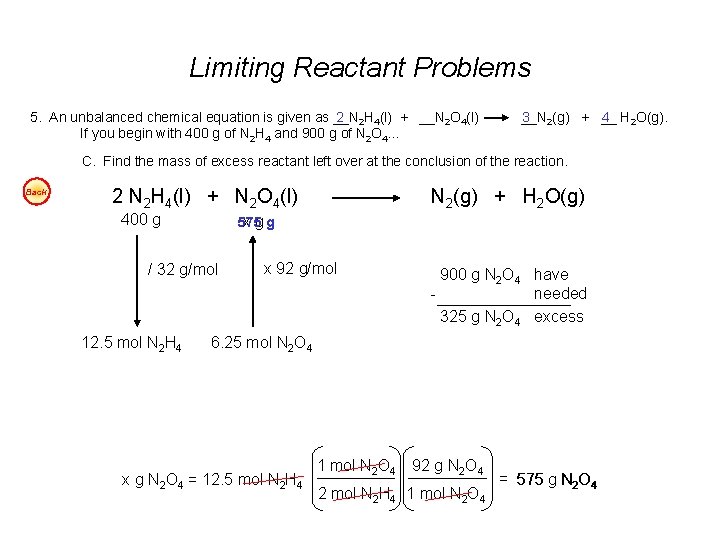
Limiting Reactant Problems 5. An unbalanced chemical equation is given as __N 2 2 H 4(l) + __N 2 O 4(l) If you begin with 400 g of N 2 H 4 and 900 g of N 2 O 4… __N 3 2(g) + __ 4 H 2 O(g). C. Find the mass of excess reactant left over at the conclusion of the reaction. Back 2 N 2 H 4(l) + N 2 O 4(l) 400 g 575 x gg / 32 g/mol 12. 5 mol N 2 H 4 N 2(g) + H 2 O(g) x 92 g/mol 900 g N 2 O 4 have needed 325 g N 2 O 4 excess 6. 25 mol N 2 O 4 x g N 2 O 4 = 12. 5 mol N 2 H 4 1 mol N 2 O 4 92 g N 2 O 4 2 mol N 2 H 4 1 mol N 2 O 4 = 575 g N 2 O 4

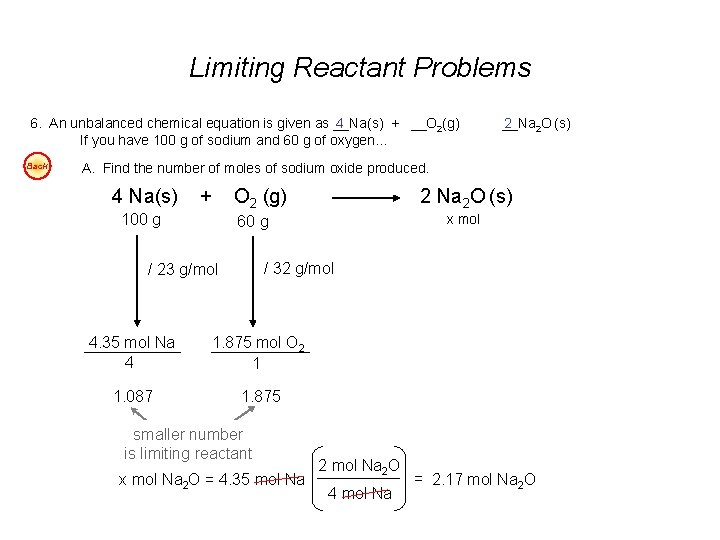
Limiting Reactant Problems 6. An unbalanced chemical equation is given as __Na(s) 4 + __O 2(g) If you have 100 g of sodium and 60 g of oxygen… Back __Na 2 2 O (s) A. Find the number of moles of sodium oxide produced. 4 Na(s) + 100 g O 2 (g) 2 Na 2 O (s) x mol 60 g / 32 g/mol / 23 g/mol 4. 35 mol Na 4 1. 875 mol O 2 1 1. 087 1. 875 smaller number is limiting reactant x mol Na 2 O = 4. 35 mol Na 2 O 4 mol Na = 2. 17 mol Na 2 O

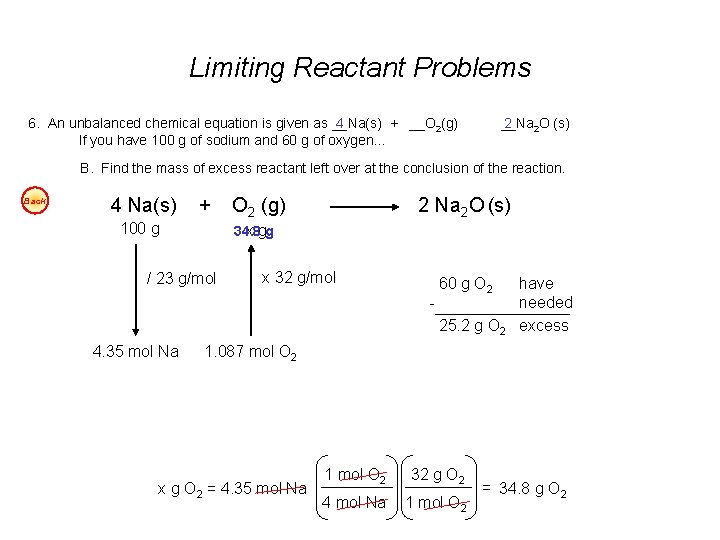
Limiting Reactant Problems 6. An unbalanced chemical equation is given as __Na(s) 4 + __O 2(g) If you have 100 g of sodium and 60 g of oxygen… __Na 2 2 O (s) B. Find the mass of excess reactant left over at the conclusion of the reaction. Back 4 Na(s) + 100 g O 2 (g) 2 Na 2 O (s) 34. 8 x gg / 23 g/mol x 32 g/mol - 60 g O 2 25. 2 g O 2 4. 35 mol Na have needed excess 1. 087 mol O 2 x g O 2 = 4. 35 mol Na 1 mol O 2 32 g O 2 4 mol Na 1 mol O 2 = 34. 8 g O 2

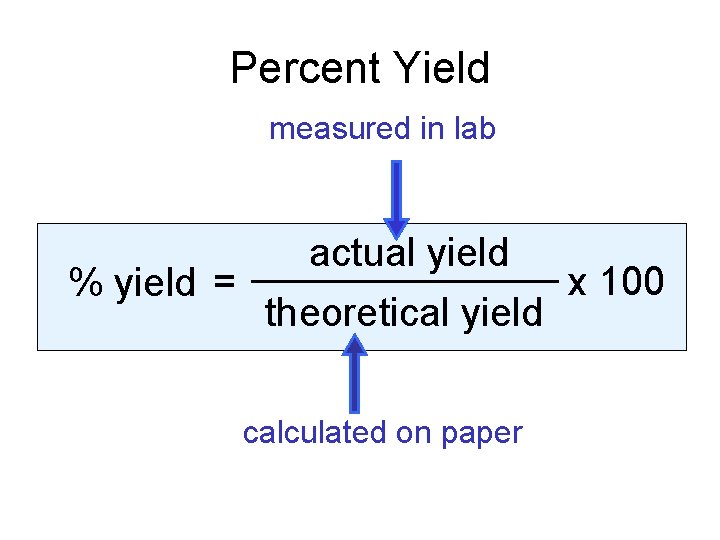
Percent Yield measured in lab % yield = actual yield theoretical yield calculated on paper x 100

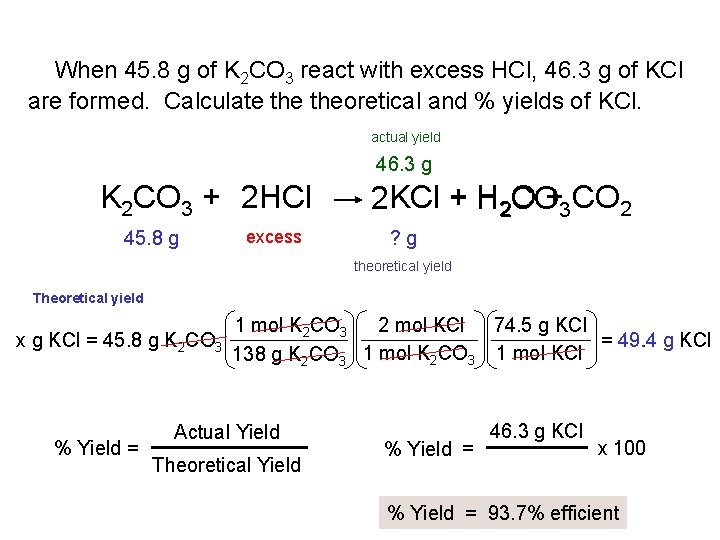
When 45. 8 g of K 2 CO 3 react with excess HCl, 46. 3 g of KCl are formed. Calculate theoretical and % yields of KCl. actual yield 46. 3 g K 2 CO 3 + 2 HCl 45. 8 g excess 2 KCl + H 2 O CO+3 CO 2 ? g theoretical yield Theoretical yield x g KCl = 45. 8 g K 2 CO 3 % Yield = 1 mol K 2 CO 3 2 mol KCl 74. 5 g KCl = 49. 4 g KCl 1 mol K CO 1 mol KCl 138 g K 2 CO 3 2 3 Actual Yield Theoretical Yield % Yield = 46. 3 g KCl x 100 % Yield = 93. 7% efficient

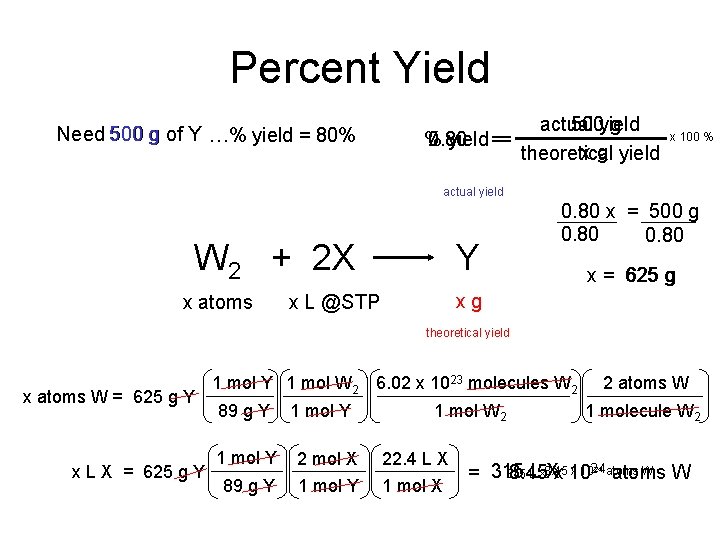
Percent Yield Need 500 g of Y …% yield = 80% actual 500 yield g 0. 80 % 80 yield == x g yield theoretical x 100 % actual yield W 2 + 2 X x atoms Y 0. 80 x = 500 g 0. 80 x = 625 g xg x L @STP theoretical yield x atoms W = 625 g Y x L X = 625 g Y 1 mol W 2 6. 02 x 1023 molecules W 2 89 g Y 1 mol W 2 1 mol Y 2 mol X 22. 4 L X 89 g Y 1 mol X 2 atoms W 1 molecule W 2 x 1024 atoms W 315 L LX 8. 45 Xx 10 = 315 8. 45 atoms W 24

Cartoon courtesy of Nearing. Zero. net

Print Copy of Lab Baking Soda Lab Na Na. HCO HCl HCO 3 + H Cl sodium bicarbonate + H 2 CO 3 hydrochloric acid sodium chloride baking soda table salt D (l) + H 2 O (g) heat CO 2 (g) actual yield ? g Na. HCO 3 + HCl 5 g excess D actual yield theoretical yield gas Na. Cl + H 2 O + CO 2 xg theoretical yield % yield = gas x 100 %

Resources - Stoichiometry Objectives - stoichiometry Objectives - mole / chemical formula Lab – nuts and bolts Lab - baking soda lab Episode 11 – The Mole S’mores activity Worksheet - careers in chemistry: farming key Worksheet - careers in chemistry: dentistry key Worksheet - easy stoichiometry Worksheet - energy Worksheet - generic Worksheet – moles and mass relationships Worksheet - percent yield Worksheet - stoichiometry problems 1 Worksheet - limiting reactants Worksheet - vocabulary Worksheet - lecture outline Textbook - questions Worksheet – visualizing limiting reactant Outline (general)