Limiting Reactants Limiting Reactant When chemists carry out














- Slides: 15


Limiting Reactants

Limiting Reactant § When chemists carry out reactions, the reactants are not usually present in stoichiometric amounts, therefore, § One reactant will be used up before the other § This one is called the limiting reactant

Limiting Reactant § § § Limits the extent of the reaction Gets used up during the reaction Determines the amount of product Determines theoretical yield Once the limiting reactant is used up, no more product can form

Excess Reactant § The reactant that is left over after the reaction is complete § Insures that one of the reactants is all used up § Speeds up the reaction

One Banana Split

Recipe for 1 Banana Split § § § 1 banana 3 cherries 3 scoops ice cream 1/2 c hot fudge 1/2 c whipped cream 1/4 c peanuts

What we have on hand § § § 5 bananas 13 cherries 161/2 scooops ice cream 2 3/4 c hot fudge 2 1/2 c peanuts 3 1/4 c whipped cream

How many Banana Splits can you make? § What is the limiting reactant? § Compare each reactant to the number of banana splits that can be made. § The reactant that creates the LEAST amount of product is limiting. § The limiting reactant cannot be determined by simply looking at the reactant amount.

§ 5 bananas X 1 banana split = 5 banana splits 1 banana § 13 Cherries X 1 Ban Spl = 4. 3 Ban Splits (LR) 3 cherries (TY) § 16. 5 Scoops X 1 Ban. Spl = 5. 50 Ban Splits 3 scoops § 2. 75 c. hot fudge X 1 Ban. Spl = 5. 50 Ban Spl 0. 5 c Hot Fudge

§ 2. 5 c peanuts X 1 ban spl = 10 Ban Spl 0. 25 c peanuts § 3. 25 c whipped crm X 1 Ban spl= 6. 5 Ban spl 0. 5 c whipped cream

How much ice cream is in excess? § 1. Identify theoretical yield (TY)using the limiting reactant (LR). § 2. Use mole equivalencies to see how much of the reactant in question is needed. § 3. Determine the amount left over by subtraction.

4. 3 Ban spl X 3 scoops = 12. 9 scoops needed 1 ban spl 16. 5 scoops available - 12. 9 scoops needed 3. 6 scoops in excess

How much whipped cream is in excess?

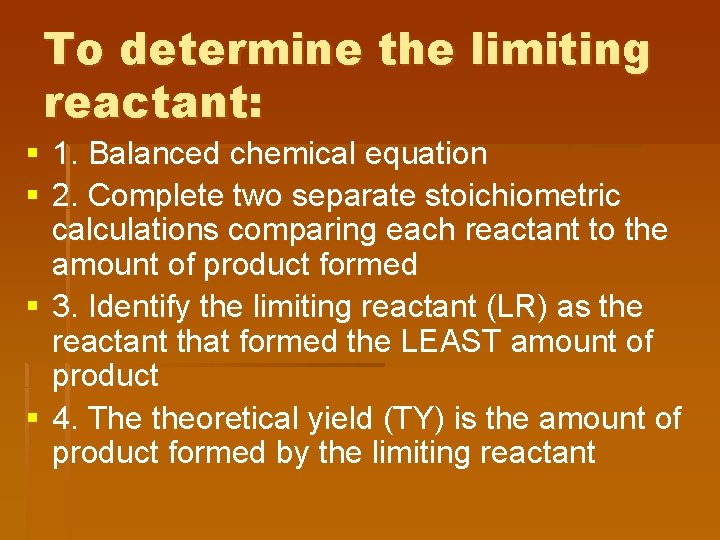
To determine the limiting reactant: § 1. Balanced chemical equation § 2. Complete two separate stoichiometric calculations comparing each reactant to the amount of product formed § 3. Identify the limiting reactant (LR) as the reactant that formed the LEAST amount of product § 4. The theoretical yield (TY) is the amount of product formed by the limiting reactant