12 3 Limiting Reagent and Percent Yield Chapter








- Slides: 54

12. 3 Limiting Reagent and Percent Yield > Chapter 12 Stoichiometry 12. 1 The Arithmetic of Equations 12. 2 Chemical Calculations 12. 3 Limiting Reagent and Percent Yield 1 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

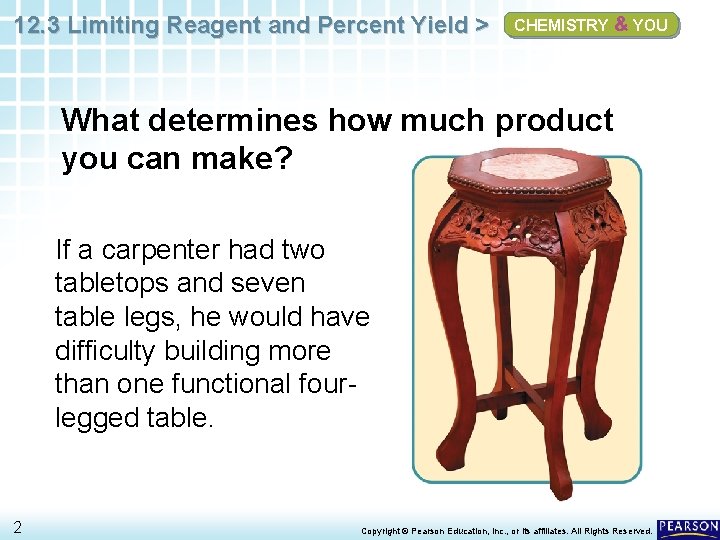
12. 3 Limiting Reagent and Percent Yield > CHEMISTRY & YOU What determines how much product you can make? If a carpenter had two tabletops and seven table legs, he would have difficulty building more than one functional fourlegged table. 2 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

12. 3 Limiting Reagent and Percent Yield > Limiting and Excess Reagents How is the amount of product in a reaction affected by an insufficient quantity of any of the reactants? 3 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

12. 3 Limiting Reagent and Percent Yield > Limiting and Excess Reagents To make tacos, you need enough meat, cheese, lettuce, tomatoes, sour cream, salsa, and seasonings. 4 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

12. 3 Limiting Reagent and Percent Yield > Limiting and Excess Reagents To make tacos, you need enough meat, cheese, lettuce, tomatoes, sour cream, salsa, and seasonings. • If you have only 2 taco shells, the quantity of taco shells will limit the number of tacos you can make. 5 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

12. 3 Limiting Reagent and Percent Yield > Limiting and Excess Reagents To make tacos, you need enough meat, cheese, lettuce, tomatoes, sour cream, salsa, and seasonings. • If you have only 2 taco shells, the quantity of taco shells will limit the number of tacos you can make. • Thus, the taco shells are the limiting ingredient. 6 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

12. 3 Limiting Reagent and Percent Yield > Limiting and Excess Reagents In a chemical reaction, an insufficient quantity of any of the reactants will limit the amount of product that forms. 7 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

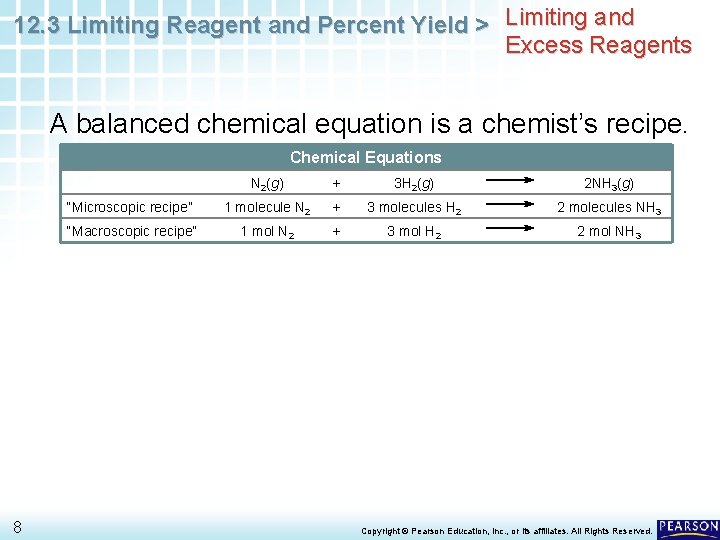
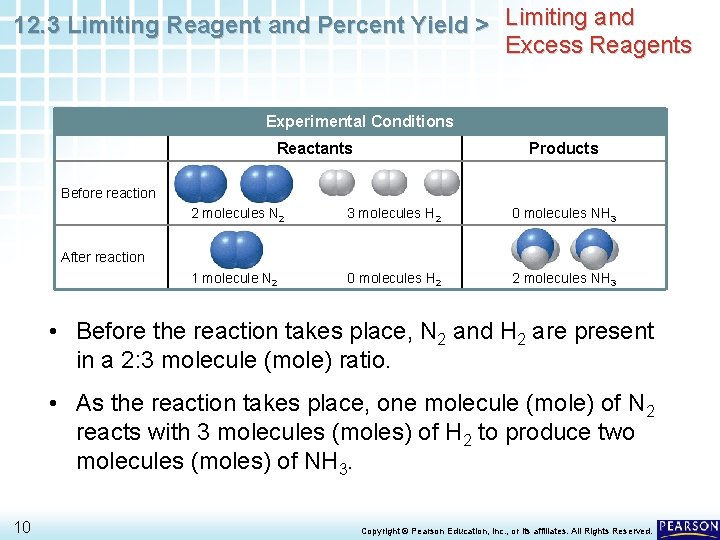
12. 3 Limiting Reagent and Percent Yield > Limiting and Excess Reagents A balanced chemical equation is a chemist’s recipe. Chemical Equations 8 N 2(g) + 3 H 2(g) 2 NH 3(g) “Microscopic recipe” 1 molecule N 2 + 3 molecules H 2 2 molecules NH 3 “Macroscopic recipe” 1 mol N 2 + 3 mol H 2 2 mol NH 3 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

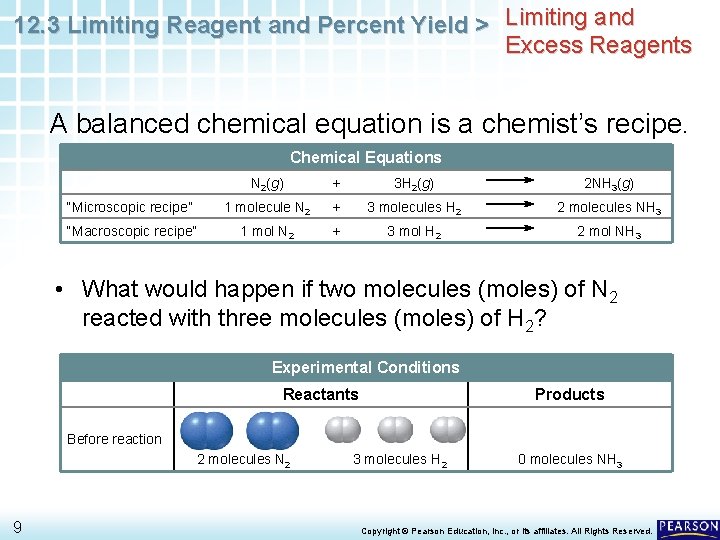
12. 3 Limiting Reagent and Percent Yield > Limiting and Excess Reagents A balanced chemical equation is a chemist’s recipe. Chemical Equations N 2(g) + 3 H 2(g) 2 NH 3(g) “Microscopic recipe” 1 molecule N 2 + 3 molecules H 2 2 molecules NH 3 “Macroscopic recipe” 1 mol N 2 + 3 mol H 2 2 mol NH 3 • What would happen if two molecules (moles) of N 2 reacted with three molecules (moles) of H 2? Experimental Conditions Reactants Products Before reaction 2 molecules N 2 9 3 molecules H 2 0 molecules NH 3 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

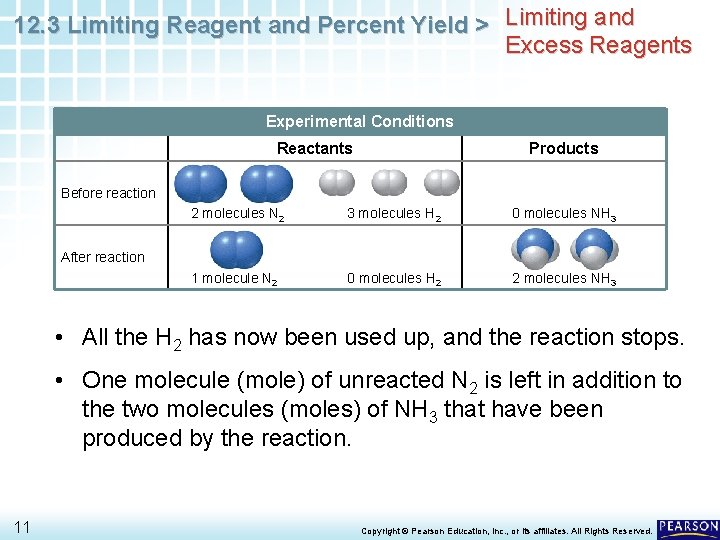
12. 3 Limiting Reagent and Percent Yield > Limiting and Excess Reagents Experimental Conditions Reactants Products Before reaction 2 molecules N 2 3 molecules H 2 0 molecules NH 3 1 molecule N 2 0 molecules H 2 2 molecules NH 3 After reaction • Before the reaction takes place, N 2 and H 2 are present in a 2: 3 molecule (mole) ratio. • As the reaction takes place, one molecule (mole) of N 2 reacts with 3 molecules (moles) of H 2 to produce two molecules (moles) of NH 3. 10 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

12. 3 Limiting Reagent and Percent Yield > Limiting and Excess Reagents Experimental Conditions Reactants Products Before reaction 2 molecules N 2 3 molecules H 2 0 molecules NH 3 1 molecule N 2 0 molecules H 2 2 molecules NH 3 After reaction • All the H 2 has now been used up, and the reaction stops. • One molecule (mole) of unreacted N 2 is left in addition to the two molecules (moles) of NH 3 that have been produced by the reaction. 11 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

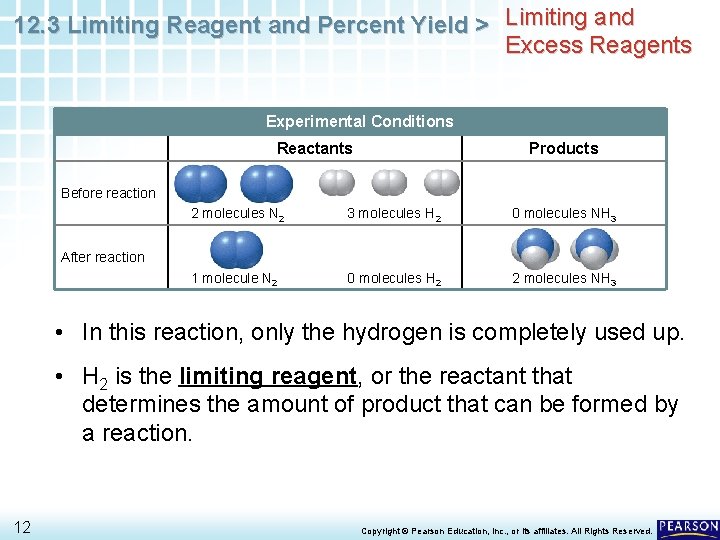
12. 3 Limiting Reagent and Percent Yield > Limiting and Excess Reagents Experimental Conditions Reactants Products Before reaction 2 molecules N 2 3 molecules H 2 0 molecules NH 3 1 molecule N 2 0 molecules H 2 2 molecules NH 3 After reaction • In this reaction, only the hydrogen is completely used up. • H 2 is the limiting reagent, or the reactant that determines the amount of product that can be formed by a reaction. 12 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

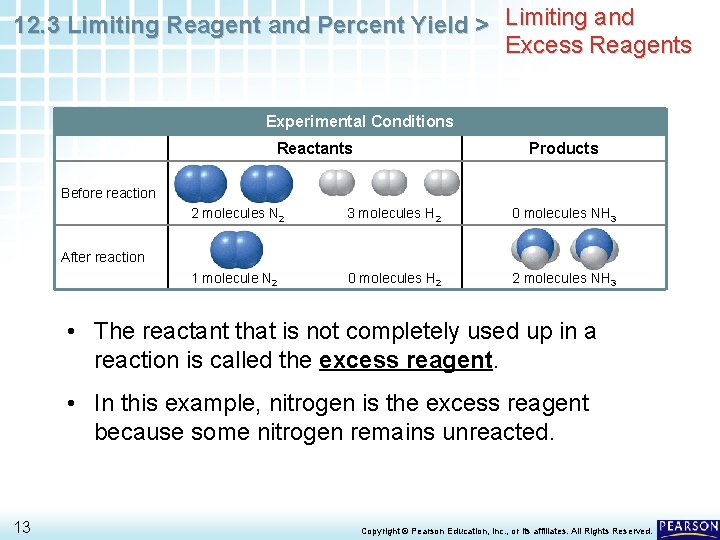
12. 3 Limiting Reagent and Percent Yield > Limiting and Excess Reagents Experimental Conditions Reactants Products Before reaction 2 molecules N 2 3 molecules H 2 0 molecules NH 3 1 molecule N 2 0 molecules H 2 2 molecules NH 3 After reaction • The reactant that is not completely used up in a reaction is called the excess reagent. • In this example, nitrogen is the excess reagent because some nitrogen remains unreacted. 13 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

12. 3 Limiting Reagent and Percent Yield > CHEMISTRY & YOU What determines how much product you can make in a chemical reaction? 14 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

12. 3 Limiting Reagent and Percent Yield > CHEMISTRY & YOU What determines how much product you can make in a chemical reaction? A limited quantity of any of the reactants that are needed to make a product will limit the amount of product that forms. 15 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

12. 3 Limiting Reagent and Percent Yield > Sample Problem 12. 8 Determining the Limiting Reagent in a Reaction Copper reacts with sulfur to form copper(I) sulfide according to the following balanced equation: 2 Cu(s) + S(s) Cu 2 S(s) What is the limiting reagent when 80. 0 g Cu reacts with 25. 0 g S? 16 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

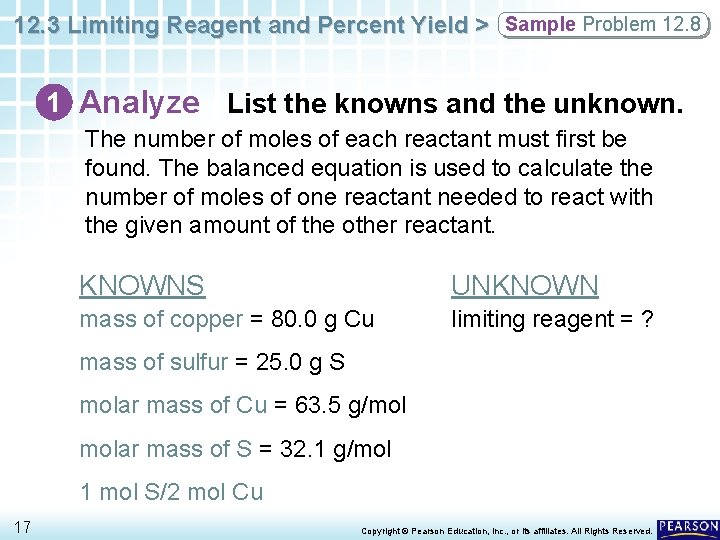
12. 3 Limiting Reagent and Percent Yield > Sample Problem 12. 8 1 Analyze List the knowns and the unknown. The number of moles of each reactant must first be found. The balanced equation is used to calculate the number of moles of one reactant needed to react with the given amount of the other reactant. KNOWNS UNKNOWN mass of copper = 80. 0 g Cu limiting reagent = ? mass of sulfur = 25. 0 g S molar mass of Cu = 63. 5 g/mol molar mass of S = 32. 1 g/mol 1 mol S/2 mol Cu 17 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

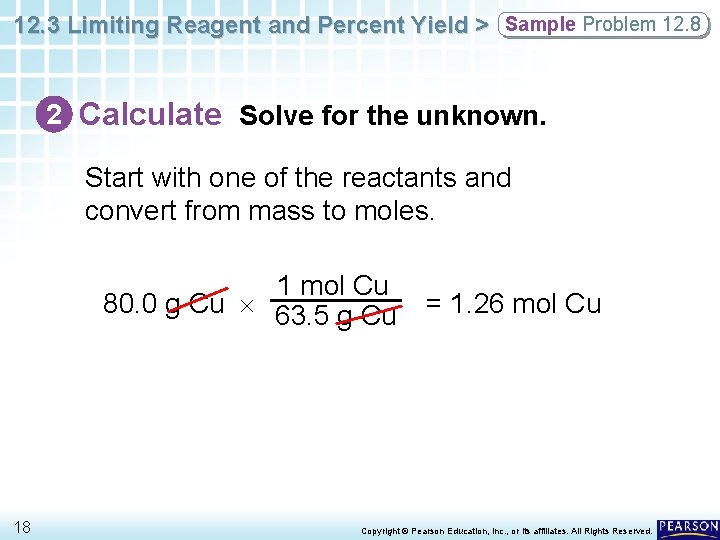
12. 3 Limiting Reagent and Percent Yield > Sample Problem 12. 8 2 Calculate Solve for the unknown. Start with one of the reactants and convert from mass to moles. 1 mol Cu 80. 0 g Cu 63. 5 g Cu 18 = 1. 26 mol Cu Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

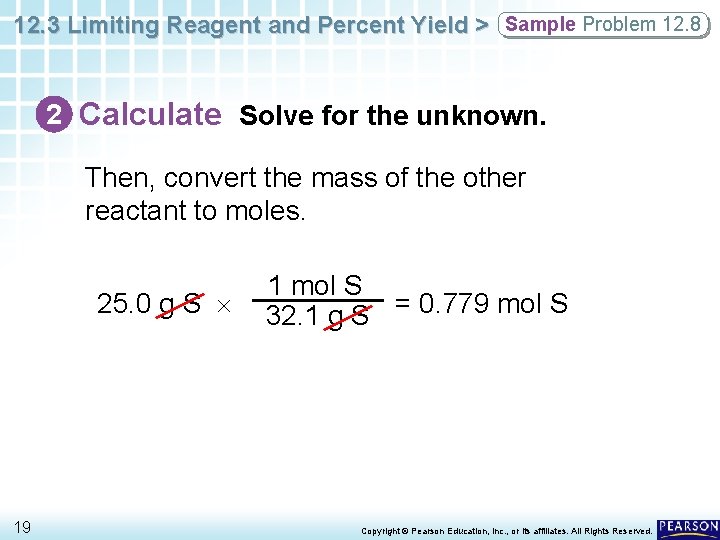
12. 3 Limiting Reagent and Percent Yield > Sample Problem 12. 8 2 Calculate Solve for the unknown. Then, convert the mass of the other reactant to moles. 25. 0 g S 19 1 mol S 32. 1 g S = 0. 779 mol S Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

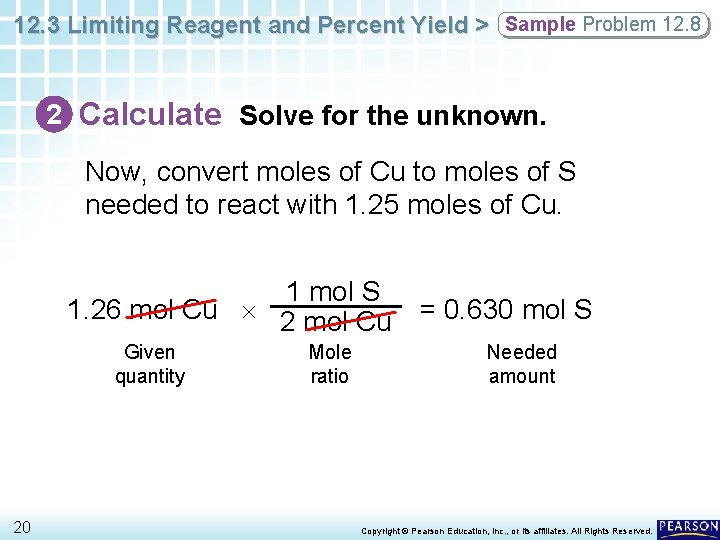
12. 3 Limiting Reagent and Percent Yield > Sample Problem 12. 8 2 Calculate Solve for the unknown. Now, convert moles of Cu to moles of S needed to react with 1. 25 moles of Cu. 1 mol S 1. 26 mol Cu 2 mol Cu = 0. 630 mol S Given quantity 20 Mole ratio Needed amount Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

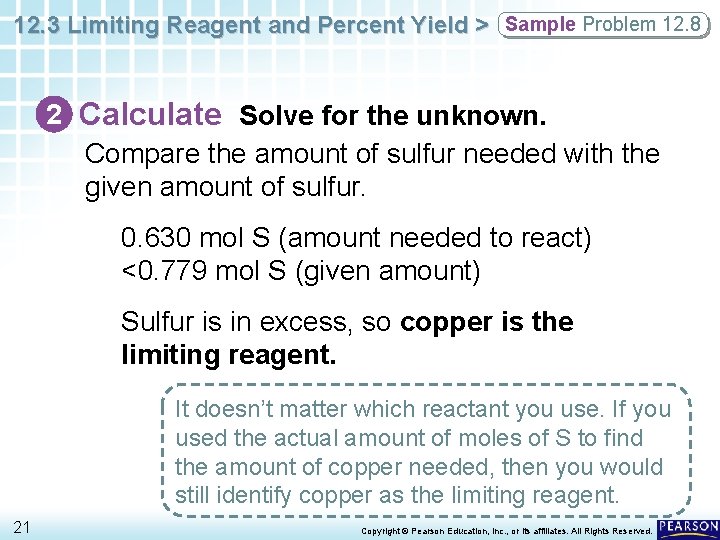
12. 3 Limiting Reagent and Percent Yield > Sample Problem 12. 8 2 Calculate Solve for the unknown. Compare the amount of sulfur needed with the given amount of sulfur. 0. 630 mol S (amount needed to react) <0. 779 mol S (given amount) Sulfur is in excess, so copper is the limiting reagent. It doesn’t matter which reactant you use. If you used the actual amount of moles of S to find the amount of copper needed, then you would still identify copper as the limiting reagent. 21 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

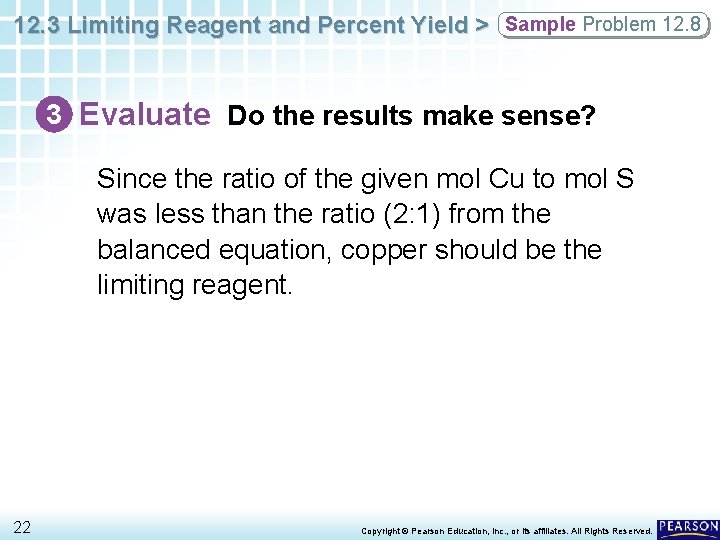
12. 3 Limiting Reagent and Percent Yield > Sample Problem 12. 8 3 Evaluate Do the results make sense? Since the ratio of the given mol Cu to mol S was less than the ratio (2: 1) from the balanced equation, copper should be the limiting reagent. 22 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

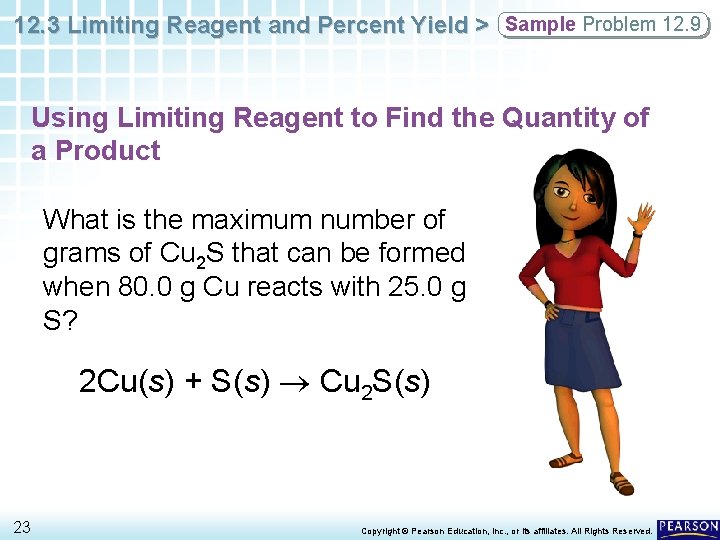
12. 3 Limiting Reagent and Percent Yield > Sample Problem 12. 9 Using Limiting Reagent to Find the Quantity of a Product What is the maximum number of grams of Cu 2 S that can be formed when 80. 0 g Cu reacts with 25. 0 g S? 2 Cu(s) + S(s) Cu 2 S(s) 23 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

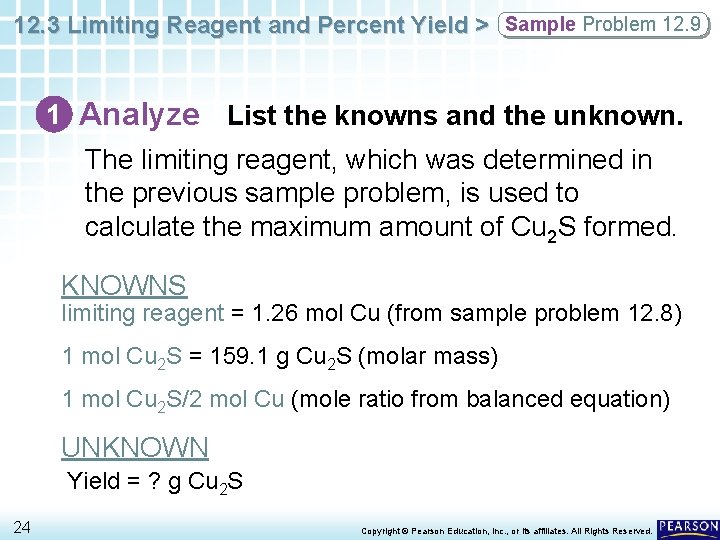
12. 3 Limiting Reagent and Percent Yield > Sample Problem 12. 9 1 Analyze List the knowns and the unknown. The limiting reagent, which was determined in the previous sample problem, is used to calculate the maximum amount of Cu 2 S formed. KNOWNS limiting reagent = 1. 26 mol Cu (from sample problem 12. 8) 1 mol Cu 2 S = 159. 1 g Cu 2 S (molar mass) 1 mol Cu 2 S/2 mol Cu (mole ratio from balanced equation) UNKNOWN Yield = ? g Cu 2 S 24 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

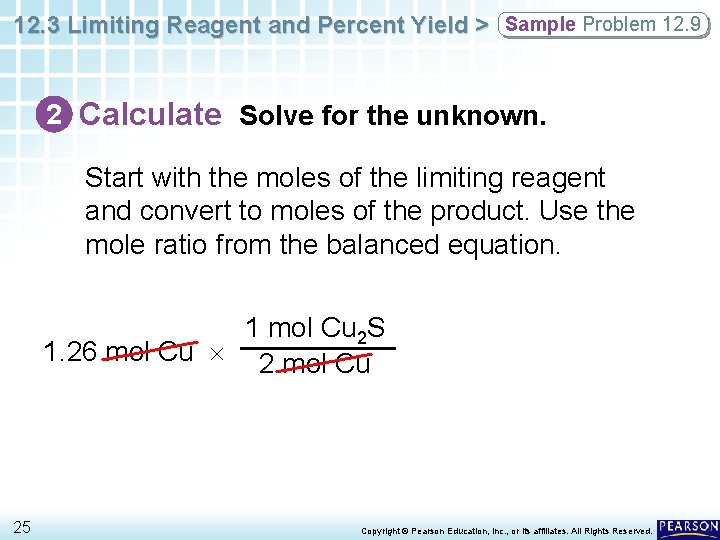
12. 3 Limiting Reagent and Percent Yield > Sample Problem 12. 9 2 Calculate Solve for the unknown. Start with the moles of the limiting reagent and convert to moles of the product. Use the mole ratio from the balanced equation. 1 mol Cu 2 S 1. 26 mol Cu 2 mol Cu 25 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

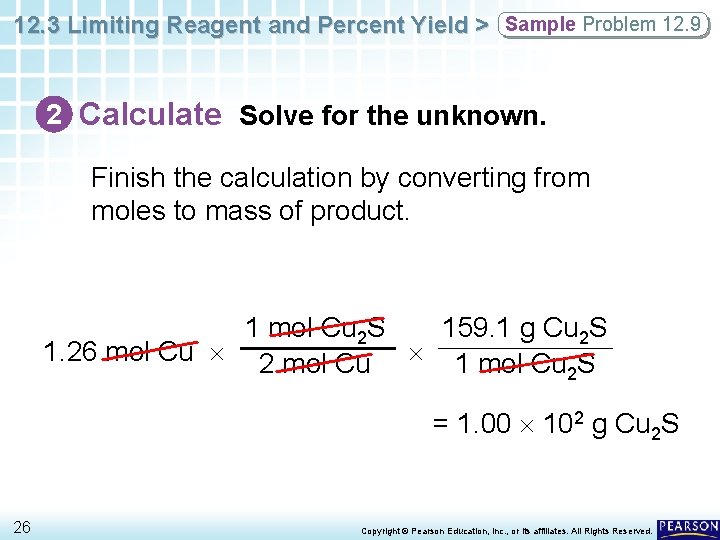
12. 3 Limiting Reagent and Percent Yield > Sample Problem 12. 9 2 Calculate Solve for the unknown. Finish the calculation by converting from moles to mass of product. 1 mol Cu 2 S 159. 1 g Cu 2 S 1. 26 mol Cu 2 mol Cu 1 mol Cu S 2 = 1. 00 102 g Cu 2 S 26 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

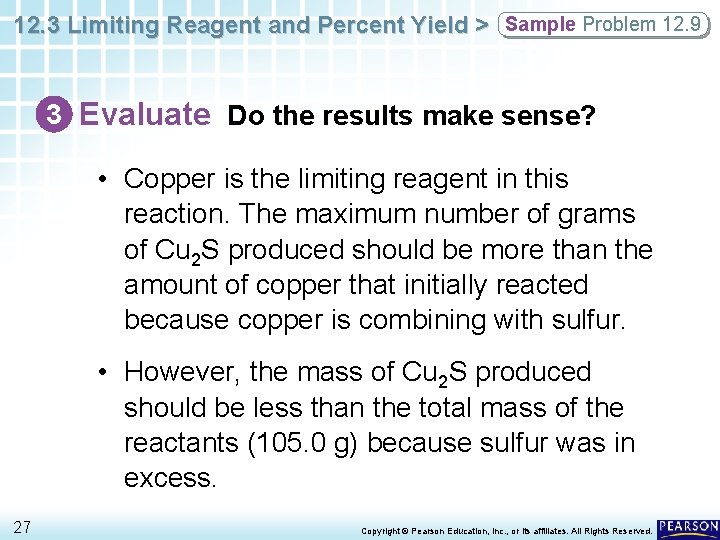
12. 3 Limiting Reagent and Percent Yield > Sample Problem 12. 9 3 Evaluate Do the results make sense? • Copper is the limiting reagent in this reaction. The maximum number of grams of Cu 2 S produced should be more than the amount of copper that initially reacted because copper is combining with sulfur. • However, the mass of Cu 2 S produced should be less than the total mass of the reactants (105. 0 g) because sulfur was in excess. 27 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

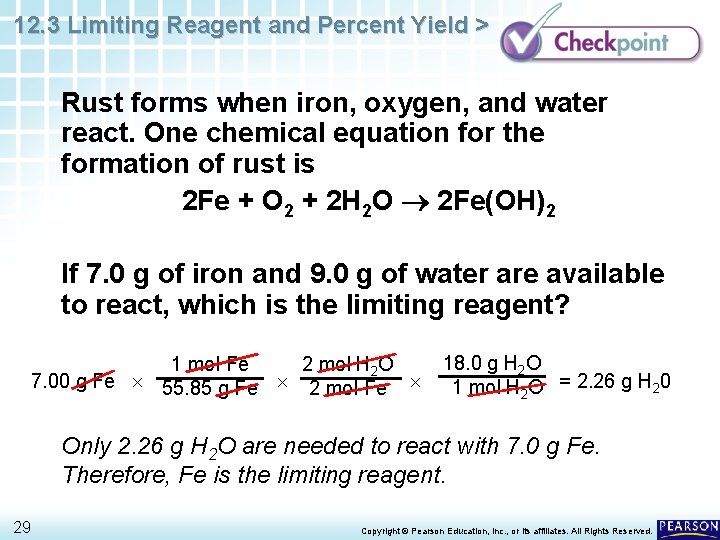
12. 3 Limiting Reagent and Percent Yield > Rust forms when iron, oxygen, and water react. One chemical equation for the formation of rust is 2 Fe + O 2 + 2 H 2 O 2 Fe(OH)2 If 7. 0 g of iron and 9. 0 g of water are available to react, which is the limiting reagent? 28 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

12. 3 Limiting Reagent and Percent Yield > Rust forms when iron, oxygen, and water react. One chemical equation for the formation of rust is 2 Fe + O 2 + 2 H 2 O 2 Fe(OH)2 If 7. 0 g of iron and 9. 0 g of water are available to react, which is the limiting reagent? 1 mol Fe 2 mol H 2 O 7. 00 g Fe 55. 85 g Fe 2 mol Fe 18. 0 g H 2 O 1 mol H 2 O = 2. 26 g H 20 Only 2. 26 g H 2 O are needed to react with 7. 0 g Fe. Therefore, Fe is the limiting reagent. 29 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

12. 3 Limiting Reagent and Percent Yield > Percent Yield What does the percent yield of a reaction measure? 30 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

12. 3 Limiting Reagent and Percent Yield > Percent Yield What does the percent yield of a reaction measure? • A batting average is actually a percent yield. 31 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

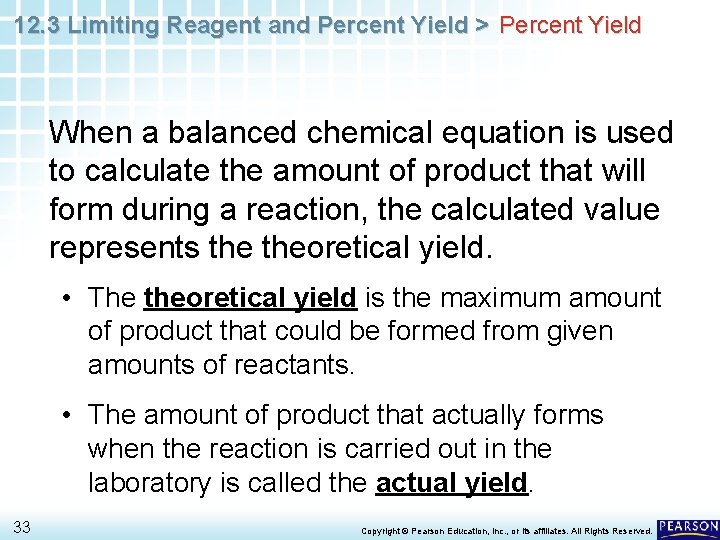
12. 3 Limiting Reagent and Percent Yield > Percent Yield When a balanced chemical equation is used to calculate the amount of product that will form during a reaction, the calculated value represents theoretical yield. 32 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

12. 3 Limiting Reagent and Percent Yield > Percent Yield When a balanced chemical equation is used to calculate the amount of product that will form during a reaction, the calculated value represents theoretical yield. • The theoretical yield is the maximum amount of product that could be formed from given amounts of reactants. • The amount of product that actually forms when the reaction is carried out in the laboratory is called the actual yield. 33 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

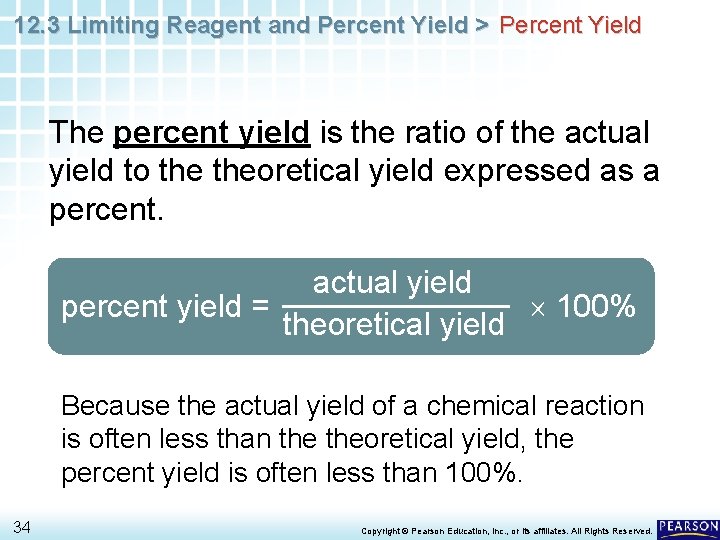
12. 3 Limiting Reagent and Percent Yield > Percent Yield The percent yield is the ratio of the actual yield to theoretical yield expressed as a percent. actual yield percent yield = 100% theoretical yield Because the actual yield of a chemical reaction is often less than theoretical yield, the percent yield is often less than 100%. 34 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

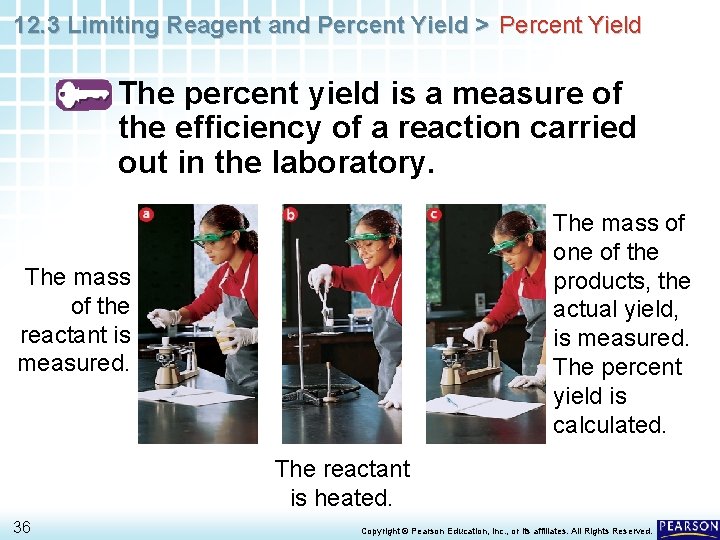
12. 3 Limiting Reagent and Percent Yield > Percent Yield The percent yield is a measure of the efficiency of a reaction carried out in the laboratory. 35 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

12. 3 Limiting Reagent and Percent Yield > Percent Yield The percent yield is a measure of the efficiency of a reaction carried out in the laboratory. The mass of one of the products, the actual yield, is measured. The percent yield is calculated. The mass of the reactant is measured. The reactant is heated. 36 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

12. 3 Limiting Reagent and Percent Yield > Percent Yield Many factors cause percent yields to be less than 100%. • Reactions do not always go to completion; when a reaction is incomplete, less than the calculated amount of product is formed. • Impure reactants and competing side reactions may cause unwanted products to form. • Actual yield can be lower than theoretical yield due to a loss of product during filtration or in transferring between containers. • If reactants or products have not been carefully measured, a percent yield of 100% is unlikely. 37 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

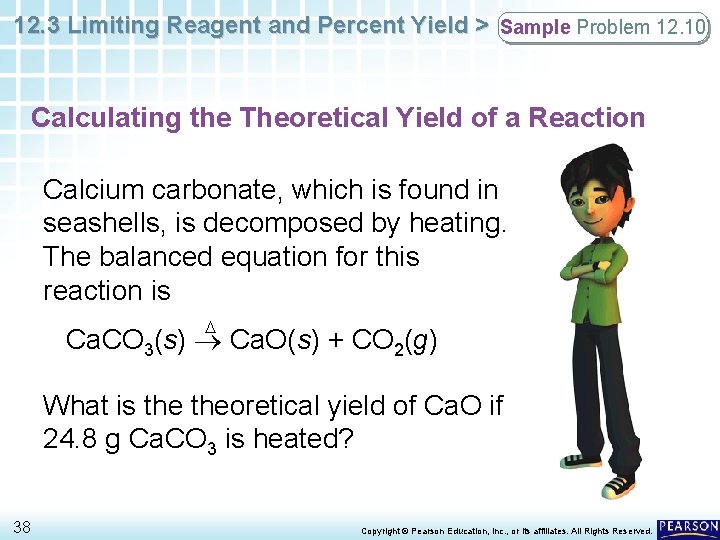
12. 3 Limiting Reagent and Percent Yield > Sample Problem 12. 10 Calculating the Theoretical Yield of a Reaction Calcium carbonate, which is found in seashells, is decomposed by heating. The balanced equation for this reaction is D Ca. CO 3(s) Ca. O(s) + CO 2(g) What is theoretical yield of Ca. O if 24. 8 g Ca. CO 3 is heated? 38 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

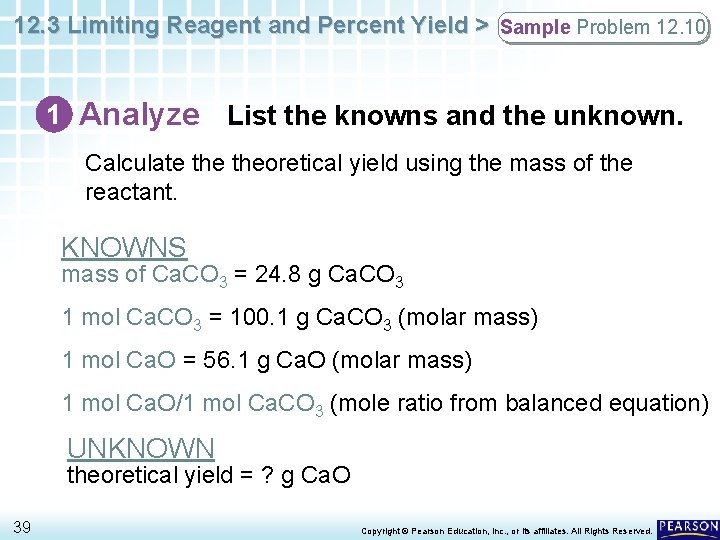
12. 3 Limiting Reagent and Percent Yield > Sample Problem 12. 10 1 Analyze List the knowns and the unknown. Calculate theoretical yield using the mass of the reactant. KNOWNS mass of Ca. CO 3 = 24. 8 g Ca. CO 3 1 mol Ca. CO 3 = 100. 1 g Ca. CO 3 (molar mass) 1 mol Ca. O = 56. 1 g Ca. O (molar mass) 1 mol Ca. O/1 mol Ca. CO 3 (mole ratio from balanced equation) UNKNOWN theoretical yield = ? g Ca. O 39 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

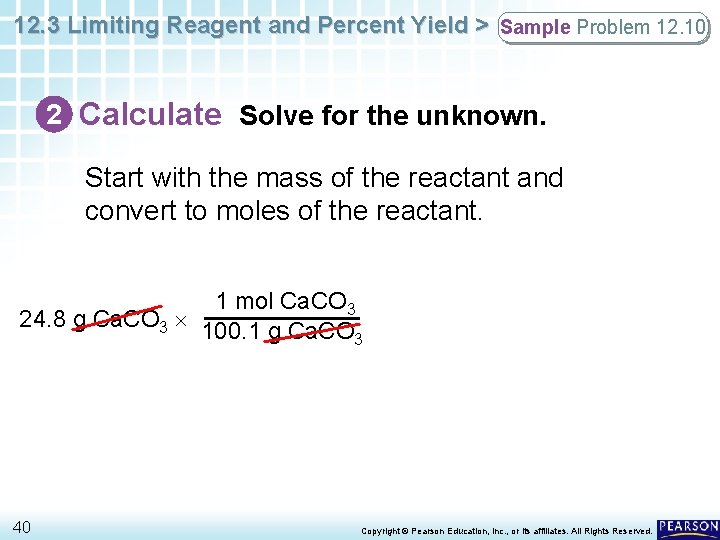
12. 3 Limiting Reagent and Percent Yield > Sample Problem 12. 10 2 Calculate Solve for the unknown. Start with the mass of the reactant and convert to moles of the reactant. 1 mol Ca. CO 3 24. 8 g Ca. CO 3 100. 1 g Ca. CO 3 40 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

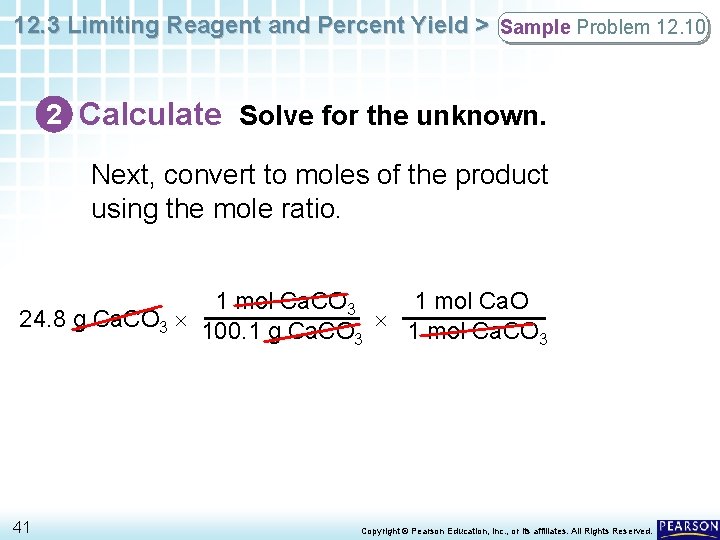
12. 3 Limiting Reagent and Percent Yield > Sample Problem 12. 10 2 Calculate Solve for the unknown. Next, convert to moles of the product using the mole ratio. 1 mol Ca. CO 3 1 mol Ca. O 24. 8 g Ca. CO 3 100. 1 g Ca. CO 1 mol Ca. CO 3 3 41 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

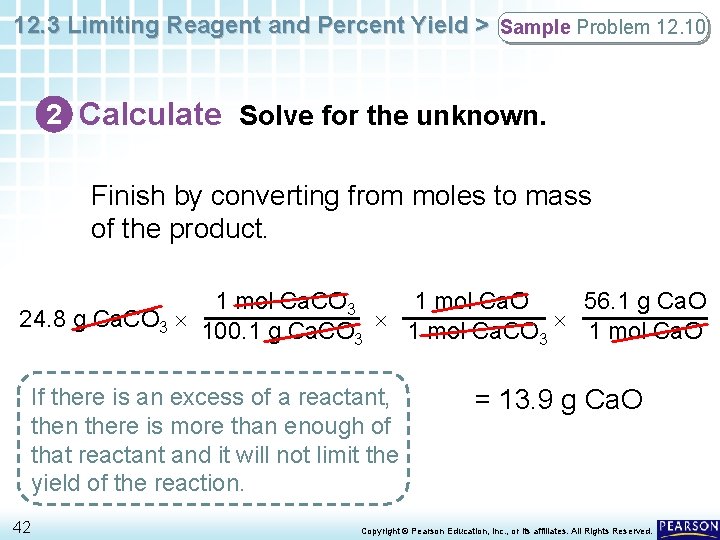
12. 3 Limiting Reagent and Percent Yield > Sample Problem 12. 10 2 Calculate Solve for the unknown. Finish by converting from moles to mass of the product. 1 mol Ca. CO 3 1 mol Ca. O 56. 1 g Ca. O 24. 8 g Ca. CO 3 100. 1 g Ca. CO 1 mol Ca. O 3 3 If there is an excess of a reactant, then there is more than enough of that reactant and it will not limit the yield of the reaction. 42 = 13. 9 g Ca. O Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

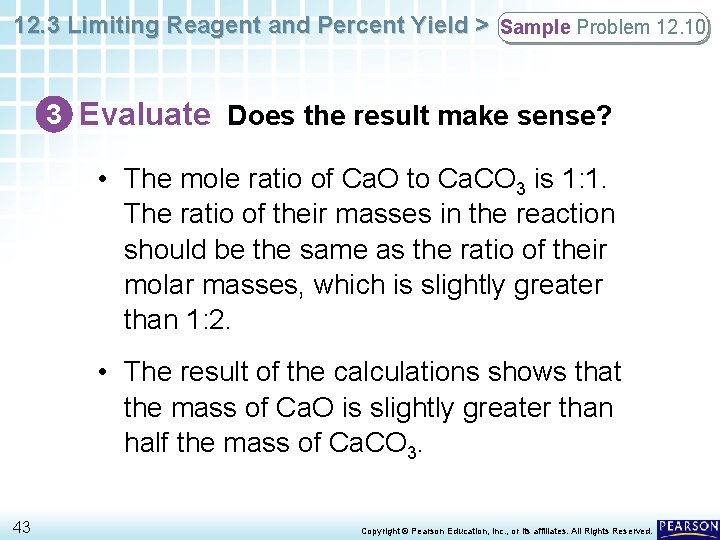
12. 3 Limiting Reagent and Percent Yield > Sample Problem 12. 10 3 Evaluate Does the result make sense? • The mole ratio of Ca. O to Ca. CO 3 is 1: 1. The ratio of their masses in the reaction should be the same as the ratio of their molar masses, which is slightly greater than 1: 2. • The result of the calculations shows that the mass of Ca. O is slightly greater than half the mass of Ca. CO 3. 43 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

12. 3 Limiting Reagent and Percent Yield > Sample Problem 12. 11 Calculating the Percent Yield of a Reaction What is the percent yield if 13. 1 g Ca. O is actually produced when 24. 8 g Ca. CO 3 is heated? Calculate theoretical yield first. Then you can calculate the percent yield. D Ca. CO 3(s) Ca. O(s) + CO 2(g) 44 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

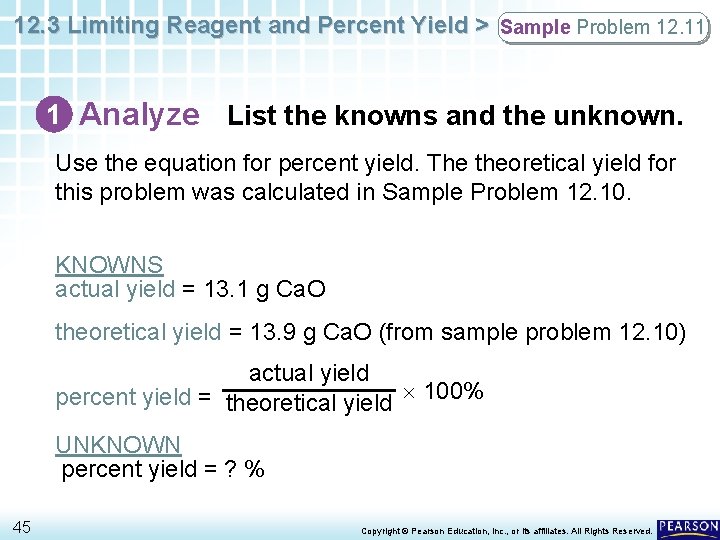
12. 3 Limiting Reagent and Percent Yield > Sample Problem 12. 11 1 Analyze List the knowns and the unknown. Use the equation for percent yield. The theoretical yield for this problem was calculated in Sample Problem 12. 10. KNOWNS actual yield = 13. 1 g Ca. O theoretical yield = 13. 9 g Ca. O (from sample problem 12. 10) actual yield percent yield = theoretical yield 100% UNKNOWN percent yield = ? % 45 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

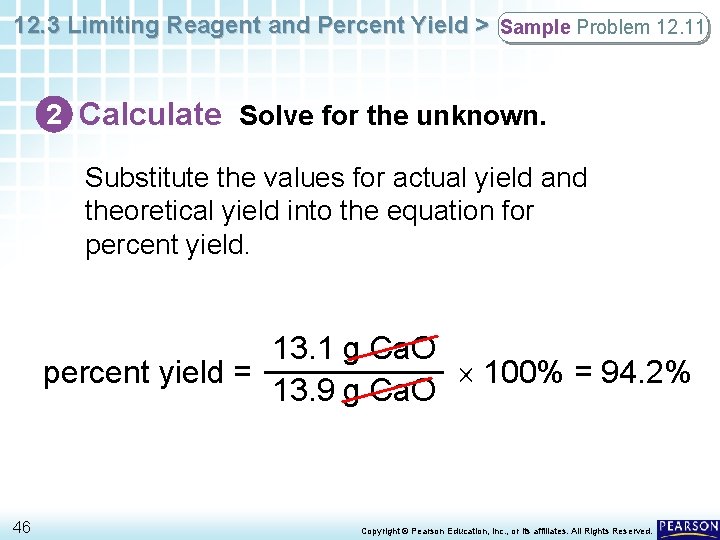
12. 3 Limiting Reagent and Percent Yield > Sample Problem 12. 11 2 Calculate Solve for the unknown. Substitute the values for actual yield and theoretical yield into the equation for percent yield. 13. 1 g Ca. O percent yield = 100% = 94. 2% 13. 9 g Ca. O 46 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

12. 3 Limiting Reagent and Percent Yield > Sample Problem 12. 11 3 Evaluate Does the result make sense? • In this example, the actual yield is slightly less than theoretical yield. • Therefore, the percent yield should be slightly less than 100%. 47 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

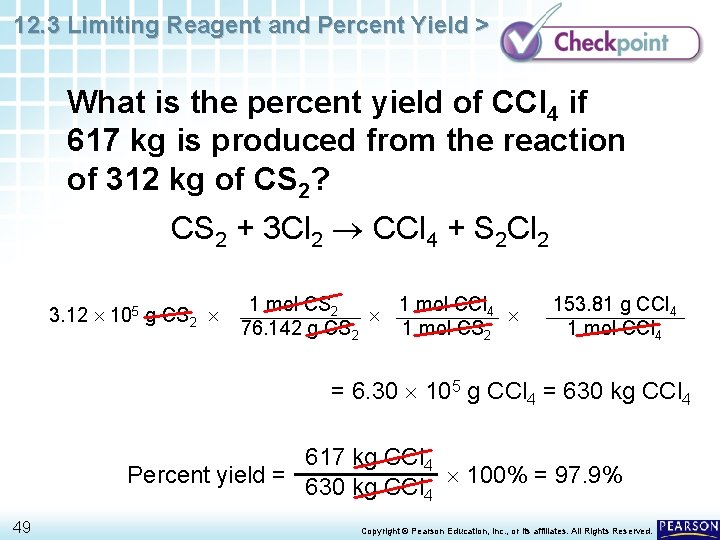
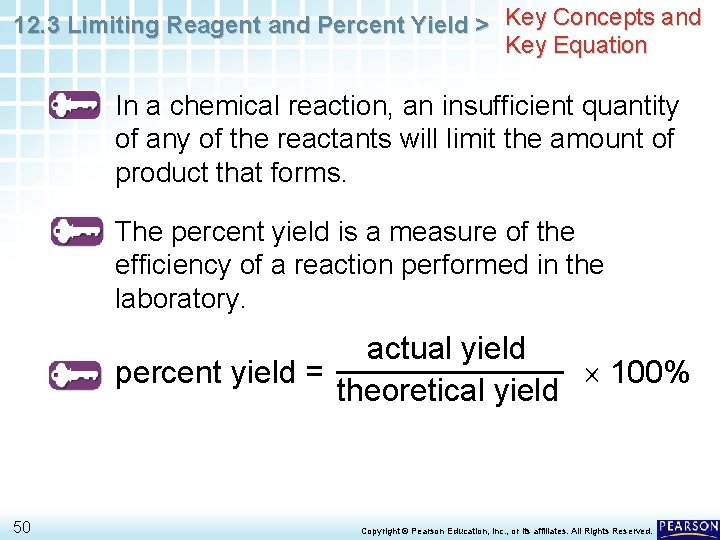
12. 3 Limiting Reagent and Percent Yield > Carbon tetrachloride, CCl 4, is a solvent that was once used in large amounts in dry cleaning. One reaction that produces carbon tetrachloride is CS 2 + 3 Cl 2 CCl 4 + S 2 Cl 2 What is the percent yield of CCl 4 if 617 kg is produced from the reaction of 312 kg of CS 2? 48 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

12. 3 Limiting Reagent and Percent Yield > What is the percent yield of CCl 4 if 617 kg is produced from the reaction of 312 kg of CS 2? CS 2 + 3 Cl 2 CCl 4 + S 2 Cl 2 3. 12 105 g CS 2 1 mol CS 2 1 mol CCl 4 76. 142 g CS 2 1 mol CS 2 153. 81 g CCl 4 1 mol CCl 4 = 6. 30 105 g CCl 4 = 630 kg CCl 4 617 kg CCl 4 Percent yield = 630 kg CCl 100% = 97. 9% 4 49 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

12. 3 Limiting Reagent and Percent Yield > Key Concepts and Key Equation In a chemical reaction, an insufficient quantity of any of the reactants will limit the amount of product that forms. The percent yield is a measure of the efficiency of a reaction performed in the laboratory. actual yield percent yield = 100% theoretical yield 50 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

12. 3 Limiting Reagent and Percent Yield > Glossary Terms • limiting reagent: any reactant that is used up first in a chemical reaction; it determines the amount of product that can be formed in the reaction • excess reagent: a reagent present in a quantity that is more than sufficient to react with a limiting reagent; any reactant that remains after the limiting reagent is used up in a chemical reaction 51 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

12. 3 Limiting Reagent and Percent Yield > Glossary Terms • theoretical yield: the amount of product that could form during a reaction calculated from a balanced chemical equation; it represents the maximum amount of product that could be formed from a given amount of reactant • actual yield: the amount of product that forms when a reaction is carried out in the laboratory • percent yield: the ratio of the actual yield to theoretical yield for a chemical reaction expressed as a percentage; a measure of the efficiency of a reaction 52 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

12. 3 Limiting Reagent and Percent Yield > BIG IDEA The Mole and Quantifying Matter The percent yield of a reaction can be calculated from the actual yield and theoretical yield of the reaction. 53 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

12. 3 Limiting Reagent and Percent Yield > END OF 12. 3 54 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.