Metric Units and Measurement Units of Measurement Why






















- Slides: 39

Metric Units and Measurement

Units of Measurement Why do we need a “standard” unit of Measurement? – Report Data that can be reproduced Base Units – Time = Seconds (s) – Length = meter (m) – Mass = kilogram (kg) – Volume = space occupied by an object • Liter (L)

Metric Units

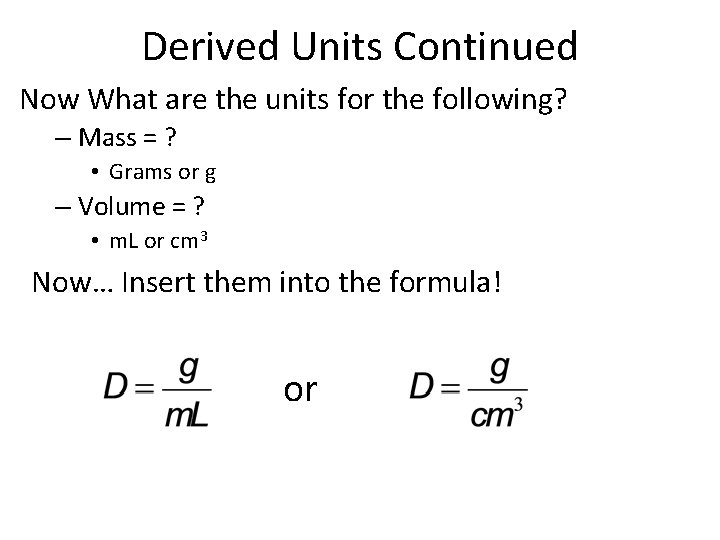
Derived Units Continued Now What are the units for the following? – Mass = ? • Grams or g – Volume = ? • m. L or cm 3 Now… Insert them into the formula! or

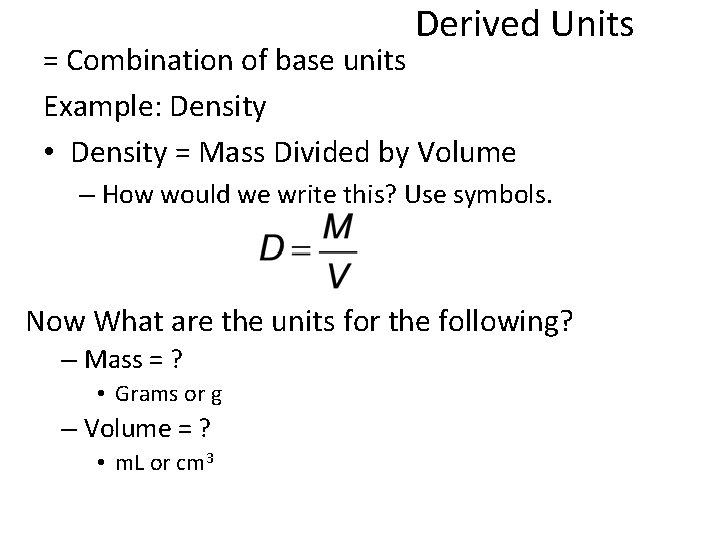
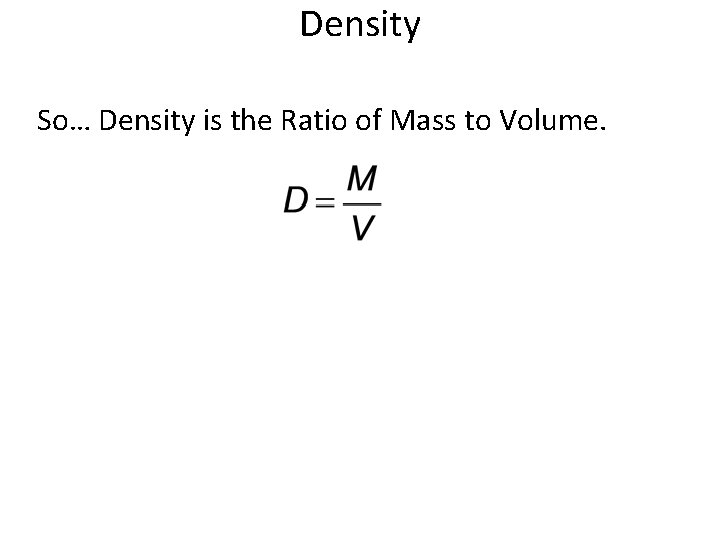
Derived Units = Combination of base units Example: Density • Density = Mass Divided by Volume – How would we write this? Use symbols. Now What are the units for the following? – Mass = ? • Grams or g – Volume = ? • m. L or cm 3

Density So… Density is the Ratio of Mass to Volume.

Determining Volume • Here is an odd shaped Object… • How would you find the volume if you couldn’t take any measurements? • Water displacement that’s how… • What happens when you get into a bath tub that is filled to the top with water? – That’s right it over flows! Why. . Water displacement

Water Displacement • Let’s take our object • And a graduated Cylinder filled with some water … enough to cover the object • … but not completely filled (remember what happened to the bath tub!)

Water Displacement and Volume • What would happen if we placed our object … into the graduated cylinder? • The Water level starts at. . – 46 m. L • Ends at 66 m. L • What’s the difference… – 20 m. L – That’s the volume of the object

Using the Density formula answer the following questions. • A piece of metal with a mass of 147 g is placed in a 50 m. L graduated cylinder. The water level rises from 20 m. L to 41 m. L. What is the density of the metal? • What is the volume of a sample that has a mass of 20 g and a density of 4 g/m. L?

Units • All measurements start with the base unit – Length is m or meters – Volume is L or liters – Mass is g or grams • How ever… what if the object is less than the base unit? • Let’s look at length or meters (m) 1 meter

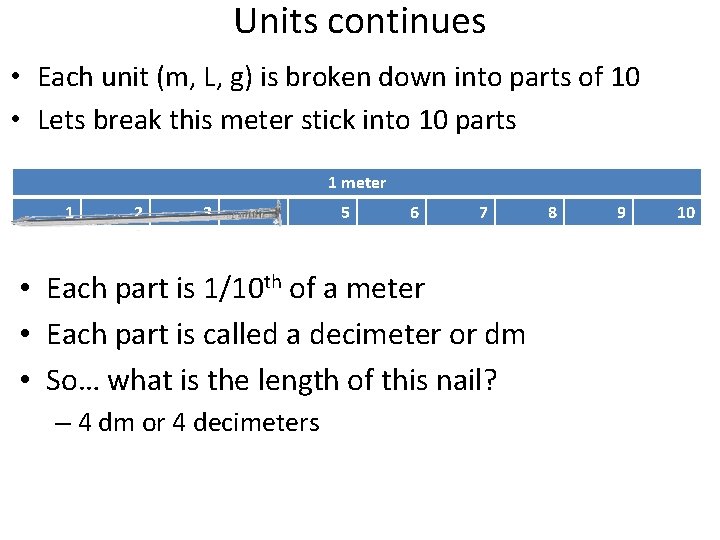
Units continues • Each unit (m, L, g) is broken down into parts of 10 • Lets break this meter stick into 10 parts 1 meter 1 2 3 4 5 6 7 • Each part is 1/10 th of a meter • Each part is called a decimeter or dm • So… what is the length of this nail? – 4 dm or 4 decimeters 8 9 10

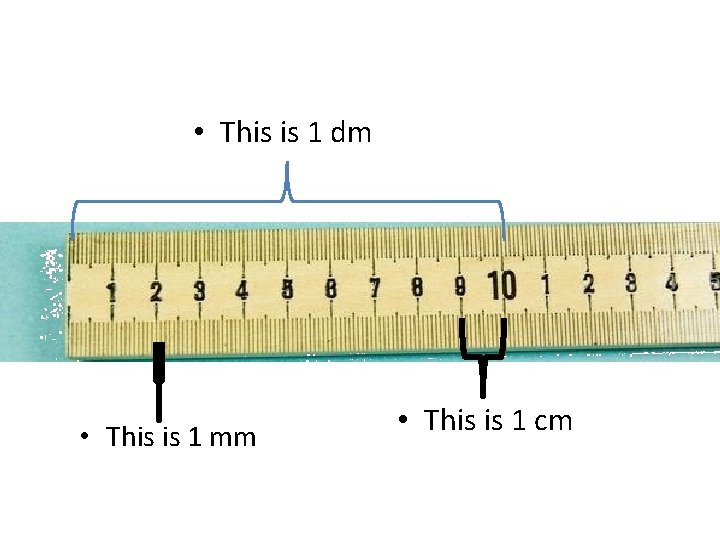
• This is 1 dm • This is 1 mm • This is 1 cm

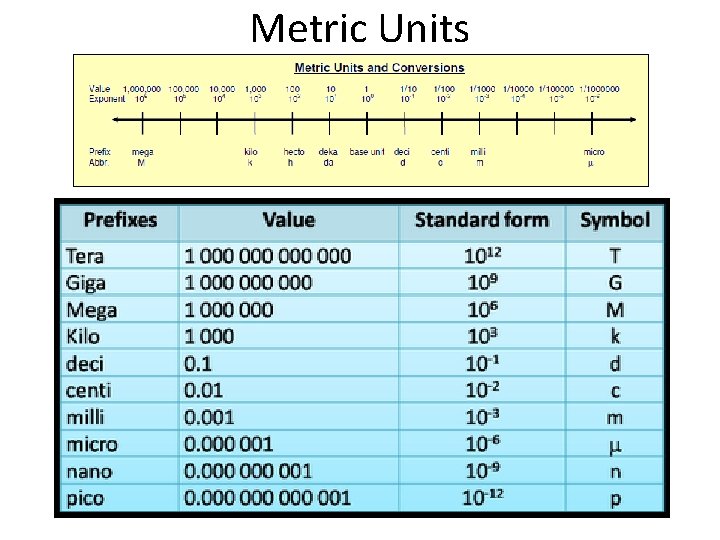
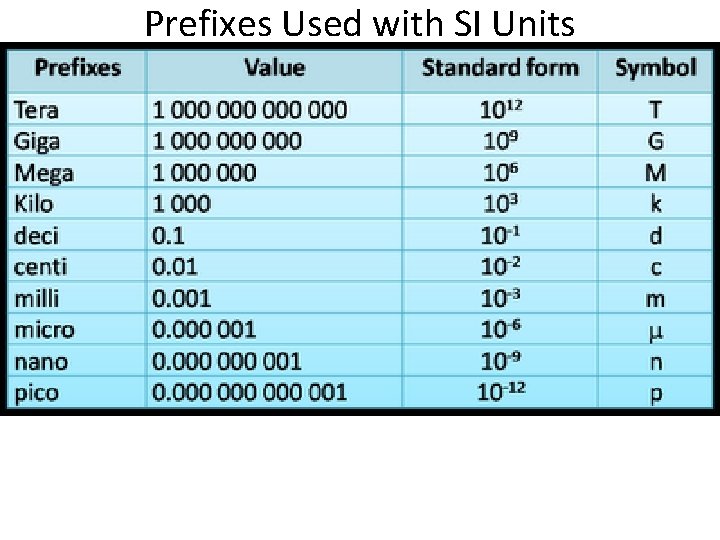
Prefixes Used with SI Units

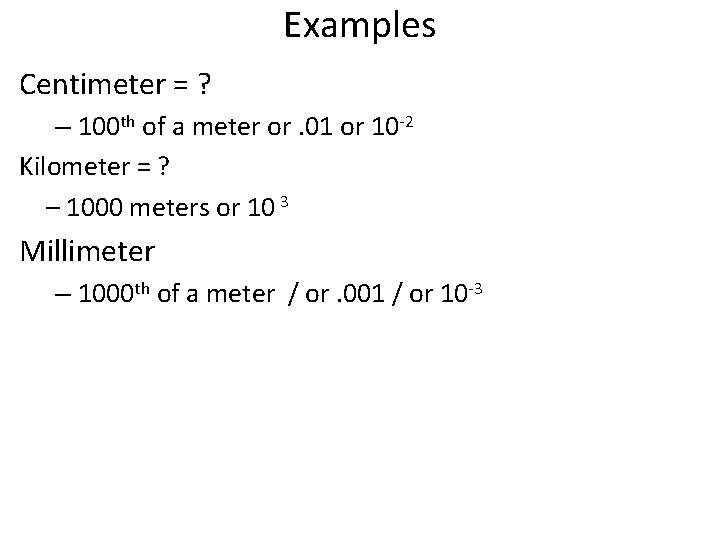
Examples Centimeter = ? – 100 th of a meter or. 01 or 10 -2 Kilometer = ? – 1000 meters or 10 3 Millimeter – 1000 th of a meter / or. 001 / or 10 -3

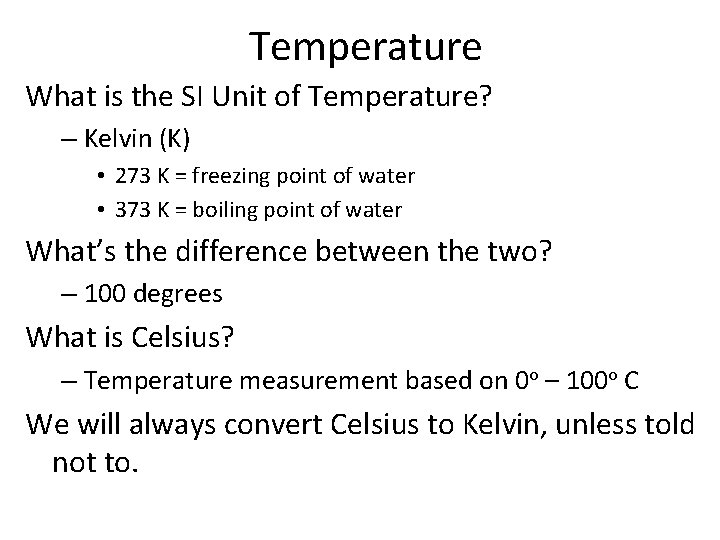
Temperature What is the SI Unit of Temperature? – Kelvin (K) • 273 K = freezing point of water • 373 K = boiling point of water What’s the difference between the two? – 100 degrees What is Celsius? – Temperature measurement based on 0 o – 100 o C We will always convert Celsius to Kelvin, unless told not to.

Converting Kelvin to Celsius Convert - Celsius to Kelvin – o. C (what you measured) + 273 K = Kelvin Convert – Kelvin to Celsius – o. K (what you measured) – 273 K = o. Celsius Convert the following to Kelvin – 357 o C – -39 o C Convert the following to Celsius – 266 K – 332 K


Activity – mini. LAB - Density • Follow the directions on page 15 of your book • We will be doing a lab write up on this lab.

NEXT CLASS: Chapter 2: 1 / 2: 2 • Homework: WB 2: 1 / 2: 2 • Quiz 3: Day 5 (Chapter 2: 1 / 2: 2)

TODAY: Chapter 2: 2 / 2: 3 DAY 5 – NO QUIZ!!! – Density LAB – Sections 2 AND 3 – Next Class Sections 2 AND 3 – HW: Sections 2 and 3 Homework: – Homework: WB 2: 2 / 2: 3 • Quiz 3: Day 6 (Chapter 2: 2 / 2: 3)

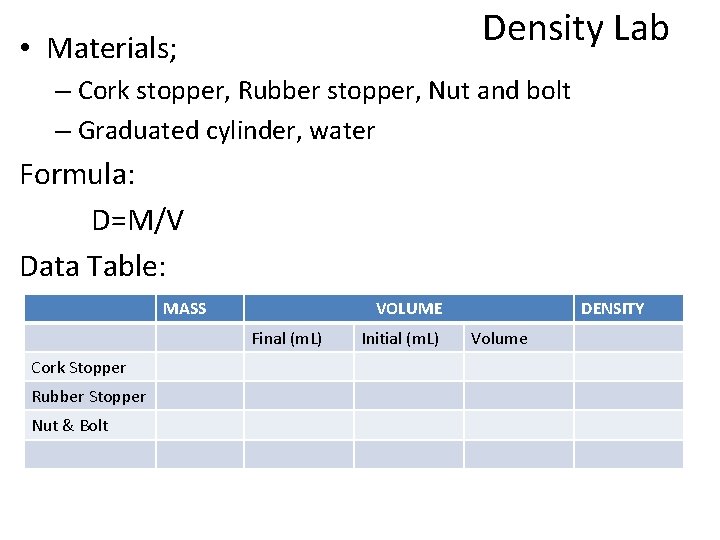
Density Lab • Materials; – Cork stopper, Rubber stopper, Nut and bolt – Graduated cylinder, water Formula: D=M/V Data Table: MASS VOLUME Final (m. L) Cork Stopper Rubber Stopper Nut & Bolt Initial (m. L) DENSITY Volume

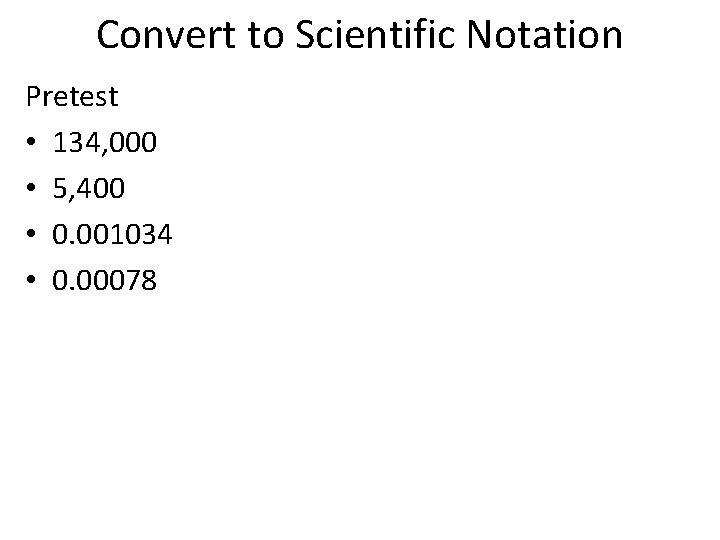
Convert to Scientific Notation Pretest • 134, 000 • 5, 400 • 0. 001034 • 0. 00078

Scientific Notation What's the goal of Scientific Notation? – Condense the number that is written Example: What would you rather write – 0. 000000000456 Or – 4. 56 x 10 -18

Rules of Scientific Notation • It’s all about the decimal point! And power of 10! Example: 645, 000 1 st… move the decimal point so one # is to the left of it 6. 45000 2 nd… place “x 10” to the right 6. 45000 x 10 3 rd… count the number of spaces you moved the decimal point. 6. 45000 X 105 Almost Done!

• 6. 45000 X 105 Now get rid of the zeros 6. 45 X 105 Rules: #1. . moving the decimal point to the left… the exponent gets bigger #2… moving the decimal point to the right… the exponent gets smaller 64. 5 X 10… what's the exponent 6. 45 X 104

Adding Exponents Rules: 1 st… exponents need to be the same. Move the decimal point until the two numbers have the same exponent 2 nd… add OR subtract the numbers… not the exponents. Example: Add the following #s…Follow the Rules 6. 45 x 105 3. 11 x 104 Answer: 6. 76 x 105

Practice problems pg 32 Addition and Subtraction • Every problem

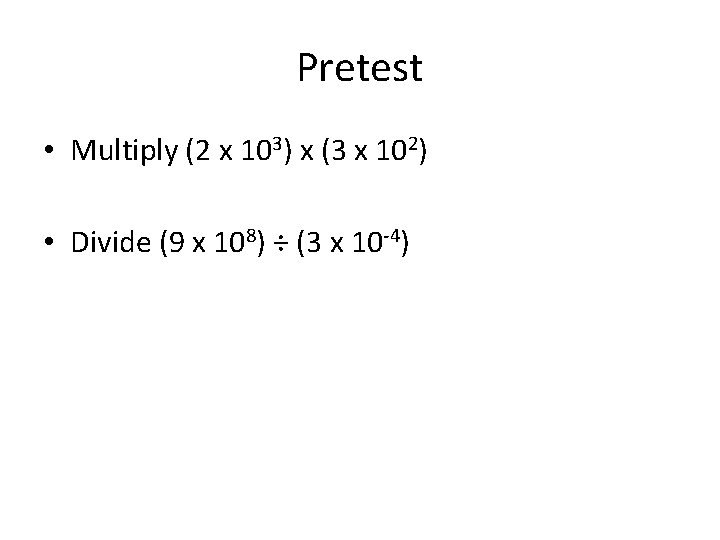
Pretest • Multiply (2 x 103) x (3 x 102) • Divide (9 x 108) ÷ (3 x 10 -4)

Multiplying / Dividing Multiplying Rules: – 1 st… multiply the numbers – 2 nd… add the exponents Dividing Rules – 1 st… divide the numbers – 2 nd… subtract the exponents

Practice Problems pg 33 Multiply (2 x 103) x (3 x 102) = Divide (9 x 108) ÷ (3 x 10 -4) =

Rules: Conversions 1 st… write down what you know 2 nd… write down what you want to know 3 rd… what conversion factor are you going to use to get there? Convert 48 km to meters (factor: 1 km=1000 meters) or =______m 48000

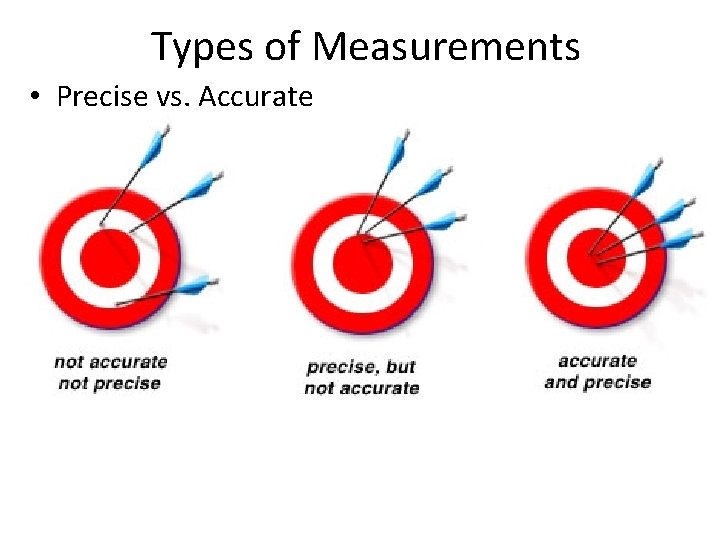
Types of Measurements • Precise vs. Accurate

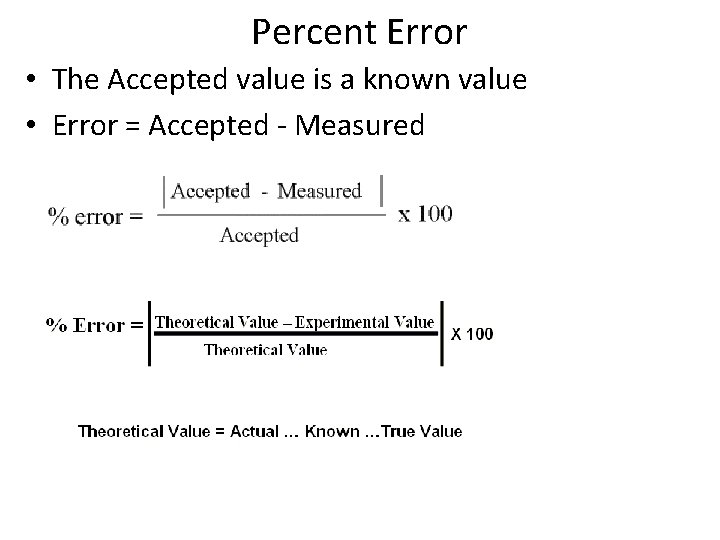
Percent Error • The Accepted value is a known value • Error = Accepted - Measured

% error example • The accepted density for copper is 8. 96 g/m. L. Calculate the percent error for each of these measurements. 1. 11% • 8. 86 g/m. L. 45% • 8. 92 g/m. L • 9. 00 g/m. L. 45% • 8. 98 g/m. L. 22%

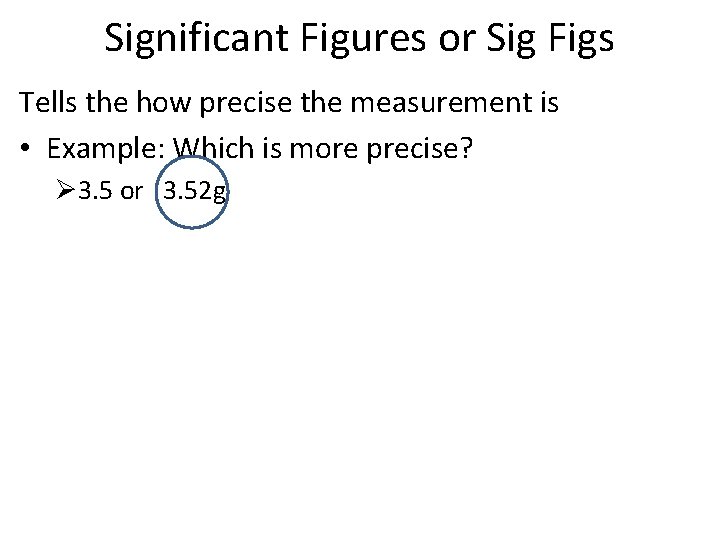
Significant Figures or Sig Figs Tells the how precise the measurement is • Example: Which is more precise? Ø 3. 5 or 3. 52 g

Rules for Sig Figs – pg. 39 1. Non-zero umbers are always significant 2. Zeros between non-zero numbers are always significant 3. All final zeros to the right of the decimal place are significant. 4. Zeros that act as placeholders are NOT significant. Convert quantities to scientific notation to remove the placeholders. 5. Counting numbers and defined constants have an infinite number of significant figures.

Examples – use your rules • Which numbers are significant? Ø 72. 3 Ø 60. 5 Ø 6. 20 Ø 0. 0253 Ø 4320 Ø 125000 • Help yourself out – convert to Scientific Notation

Rounding Numbers – pg 40 1. If the digit to the immediate right of the last sig fig is less than five, do not change the last sig fig. 2. If the digit to the immediate right of the last sig fig is greater than five, round up the last sig fig 3. If the digit to the immediate right of the last sig fig is equal to five and is followed by a nonzero digit, round up the last sig fig. 4. If the digit to the immediate right of the last sig fig is equal to five and is not followed by a nonzero digit, look at the last sig fig. if it is an odd digit, round it up. If it is an even digit, do not round up
 Appropriate metric unit of typical room height
Appropriate metric unit of typical room height Metric system table
Metric system table Metric system table
Metric system table Who uses imperial system
Who uses imperial system Ladder method conversions
Ladder method conversions Hey bye bye
Hey bye bye Metric mania
Metric mania Metric system review
Metric system review English and metric units
English and metric units Length mass and capacity
Length mass and capacity Customary and metric units
Customary and metric units Converting customary units
Converting customary units Metric measurement virtual lab
Metric measurement virtual lab Metric system of measurement
Metric system of measurement Metric measurement images
Metric measurement images What are the 7 metric units?
What are the 7 metric units? Base units for metric system
Base units for metric system Milli centi deci chart
Milli centi deci chart Metric units of capacity
Metric units of capacity Metric system conversion
Metric system conversion Metric units chemistry
Metric units chemistry The standard metric units of density are:
The standard metric units of density are: Advantages of metric system
Advantages of metric system Metric ladder chart
Metric ladder chart What is the ladder method for conversions
What is the ladder method for conversions What is the volume of this aquarium
What is the volume of this aquarium Welding marks on drawings
Welding marks on drawings English linear measurements
English linear measurements English vs metric system
English vs metric system Converting length
Converting length Dont ask why why why
Dont ask why why why Units, physical quantities, and vectors
Units, physical quantities, and vectors Measurement units standards and services department
Measurement units standards and services department Gallon man
Gallon man Unit of measurement for momentum
Unit of measurement for momentum The customary system
The customary system Customary units of measurement
Customary units of measurement Units of measurement microscope
Units of measurement microscope Opisometer is used to measure
Opisometer is used to measure Xeomin reconstitution chart
Xeomin reconstitution chart