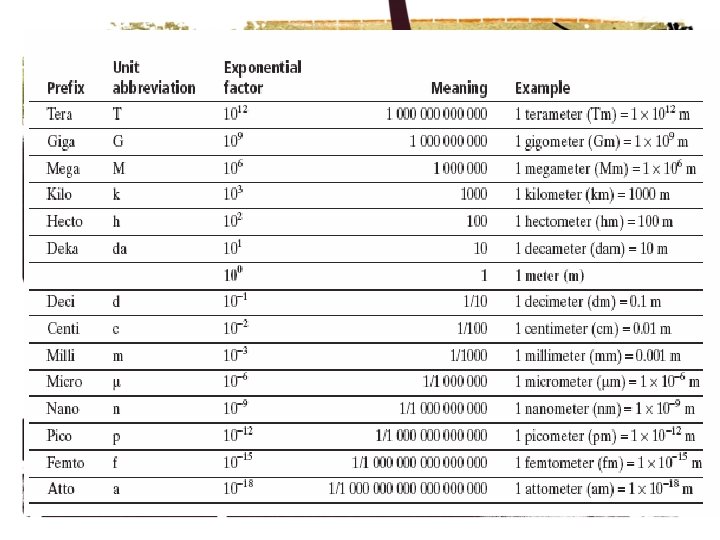
Unit Conversion of Measurements Derived Unit A derived













- Slides: 21

Unit Conversion of Measurements

Derived Unit • A derived unit is a combination of the base units such as area (_m 2_), volume, pressure, weight, force, and speed.

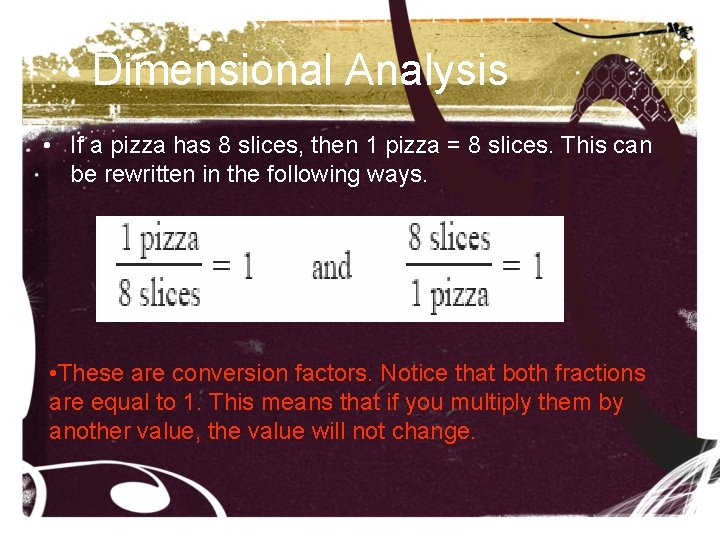
Dimensional Analysis Sometimes the unit in which a quantity was measured must be converted to a different unit so it can be used in mathematical operations. Suppose you have half a pizza and you want to know how many slices you have. Pizza and slices cannot be compared because they are different units. To compare them, you need a conversion factor. If a pizza has 8 slices, then 1 pizza = 8 slices. This can be rewritten in the following ways.

Dimensional Analysis • If a pizza has 8 slices, then 1 pizza = 8 slices. This can be rewritten in the following ways. • These are conversion factors. Notice that both fractions are equal to 1. This means that if you multiply them by another value, the value will not change.

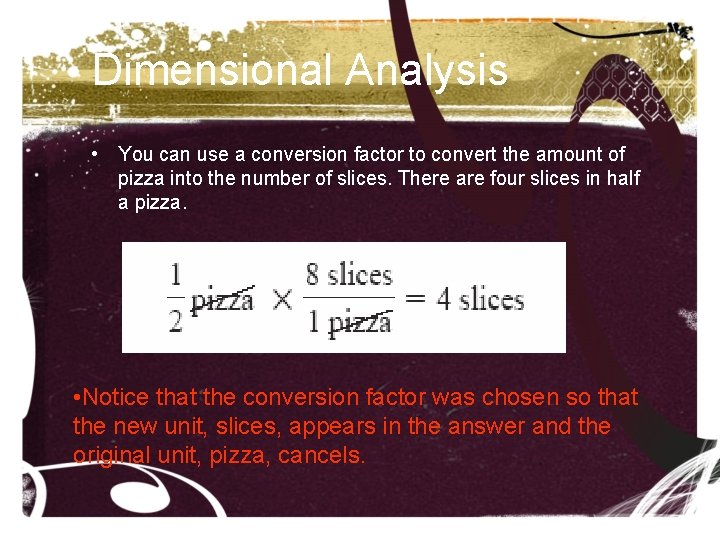
Dimensional Analysis • You can use a conversion factor to convert the amount of pizza into the number of slices. There are four slices in half a pizza. • Notice that the conversion factor was chosen so that the new unit, slices, appears in the answer and the original unit, pizza, cancels.

Simple Conversions How many meters are in 54 cm?

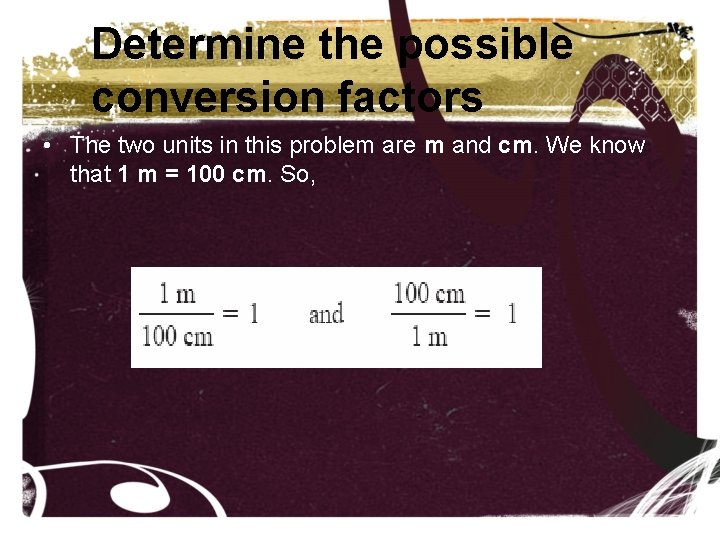
Determine the possible conversion factors • The two units in this problem are m and cm. We know that 1 m = 100 cm. So,

Decide which conversion factor will give the desired unit for the answer • The answer needs to be in m. To get m we need to use the conversion factor that has m in the numerator and divides by cm (to cancel cm in the original value).

Multiply the original value by the conversion factor.

A roll of copper wire contains 15 m of wire. What is the length of the wire in centimeters? 1. possible conversion factors : 1 m/ 100 cm or 100 cm/ 1 m 2. conversion factor used: 100 cm / 1 m 3. multiply original value: 15 m x 100 cm/ 1 m = 1 500 cm

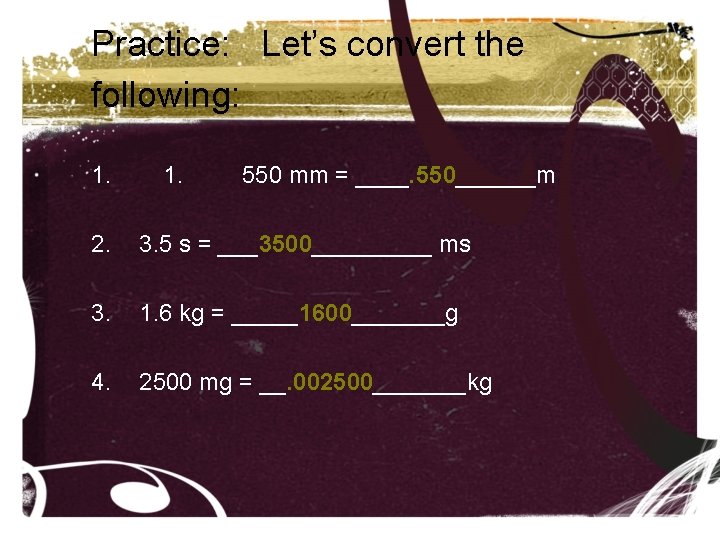
Practice: Let’s convert the following: 1. 550 mm = ____. 550______m 2. 3. 5 s = ___3500_____ ms 3. 1. 6 kg = _____1600_______g 4. 2500 mg = __. 002500_______kg


Density and Specific Gravity Would the density of a person by the same on the surface of the earth and on the surface of the moon?

• Density the amount of matter in an object per unit volume • As the temperature of a material increases, the density tends to decrease. • In the lab, the density of an irregularly shaped object is found by “water displacement”

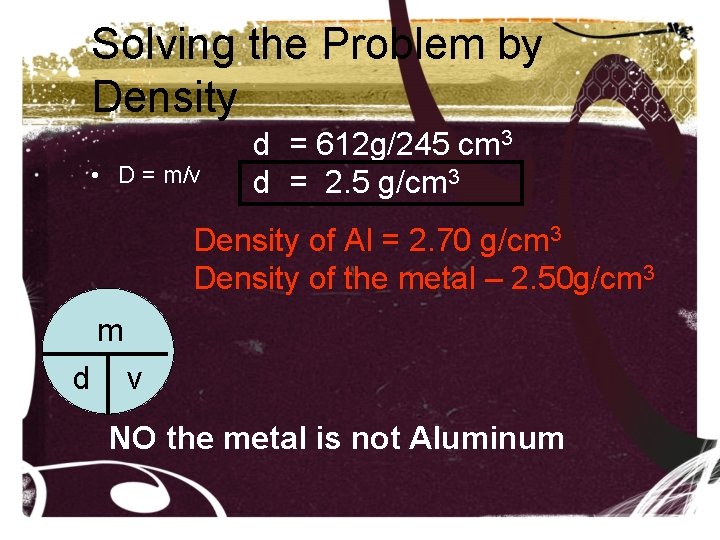
Density • D = m/v • m = mass (in grams) • V = volume (in cm 3, ml or if a gas L) • D = density (g/cm 3 , solid, • g/ml liquid, • g/L, gas) • A student finds a shiny piece of metal she thinks is aluminum. In the lab, she determines that the metal has a volume of 245 cm 3 and a mass of 612 g. Is the metal aluminum?

Solving the Problem by Density • D = m/v d = 612 g/245 cm 3 d = 2. 5 g/cm 3 Density of Al = 2. 70 g/cm 3 Density of the metal – 2. 50 g/cm 3 m d v NO the metal is not Aluminum

PROBLEM • A plastic ball with a volume of 19. 7 cm 3 has a mass of 15. 8 g. Would the ball float or sink in a container of gasoline? (density of gas 0. 66 -0. 69 g/cm 3) • A piece of iron has a mass of 2. 25 g. What is its volume?

Specific Gravity • Specific gravity – a ratio of the density of a substance to the density of another substance (usually water) at the same temperature • Density of water at 4ºC = 1. 0 g/cm 3 Gravity = density of substance (Specific g/cm 3) density of water (g/cm 3)

Specific Gravity • Specific gravity has no units – b/c they cancel out • At 4ºC what can you say about the density of an object and its specific gravity? • Specific gravity is measured using a “hydrometer”

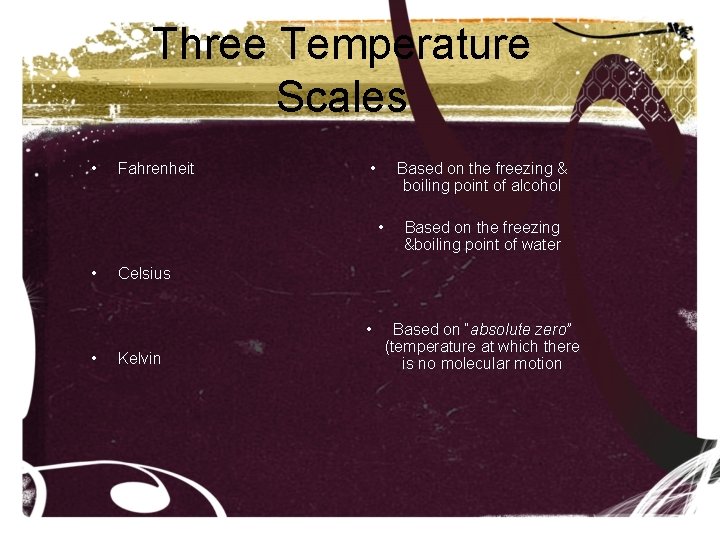
Three Temperature Scales • Fahrenheit • Based on the freezing & boiling point of alcohol • • Celsius • • Based on the freezing &boiling point of water Kelvin Based on “absolute zero” (temperature at which there is no molecular motion

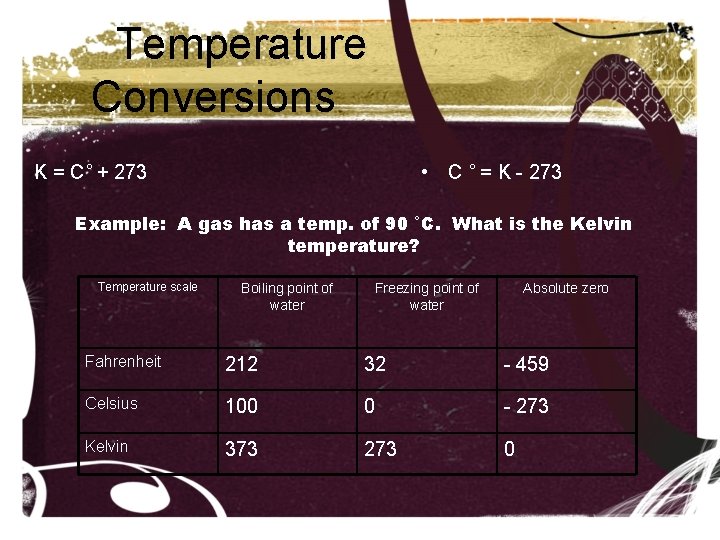
Temperature Conversions • K = C˚ + 273 • C ˚ = K - 273 Example: A gas has a temp. of 90 ˚C. What is the Kelvin temperature? Temperature scale Boiling point of water Freezing point of water Absolute zero Fahrenheit 212 32 - 459 Celsius 100 0 - 273 Kelvin 373 273 0