2 The Chemistry and Energy of Life Concept



















- Slides: 70

2 The Chemistry and Energy of Life

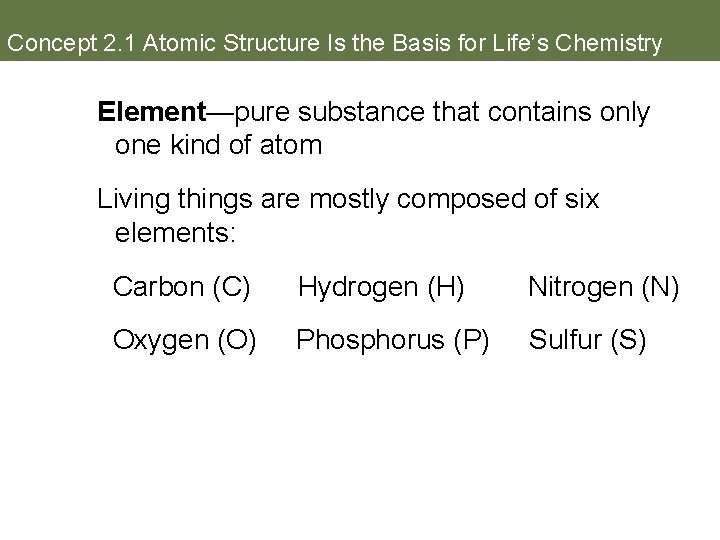
Concept 2. 1 Atomic Structure Is the Basis for Life’s Chemistry Element—pure substance that contains only one kind of atom Living things are mostly composed of six elements: Carbon (C) Hydrogen (H) Nitrogen (N) Oxygen (O) Phosphorus (P) Sulfur (S)

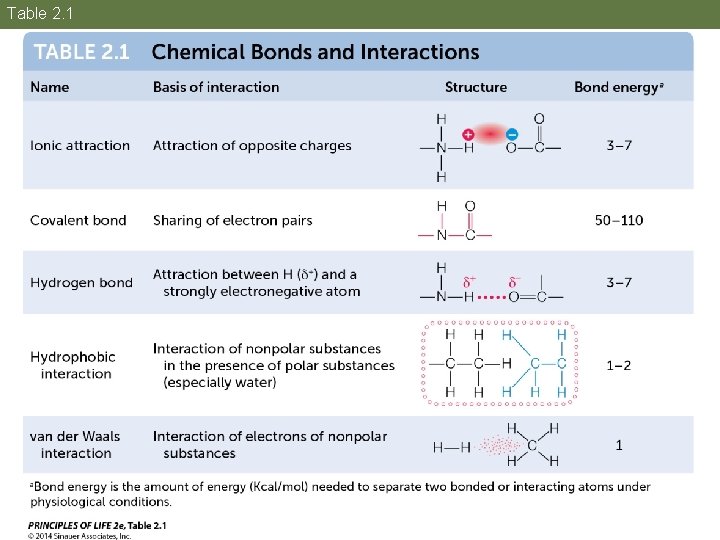
Concept 2. 2 Atoms Interact and Form Molecules A chemical bond is an attractive force that links atoms together in molecules. There are several kinds of chemical bonds.

Table 2. 1

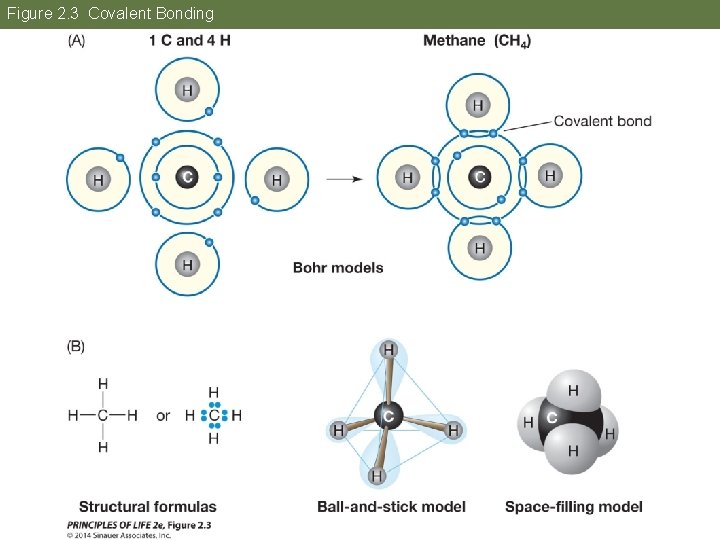
Concept 2. 2 Atoms Interact and Form Molecules Covalent bonds form when two atoms share pairs of electrons. The atoms attain stability by having full outer shells. Each atom contributes one member of the electron pair.

Figure 2. 3 Covalent Bonding

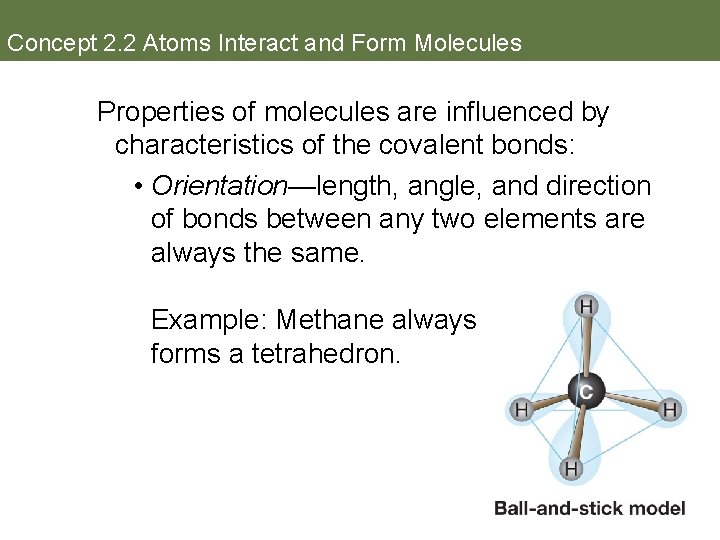
Concept 2. 2 Atoms Interact and Form Molecules Properties of molecules are influenced by characteristics of the covalent bonds: • Orientation—length, angle, and direction of bonds between any two elements are always the same. Example: Methane always forms a tetrahedron.

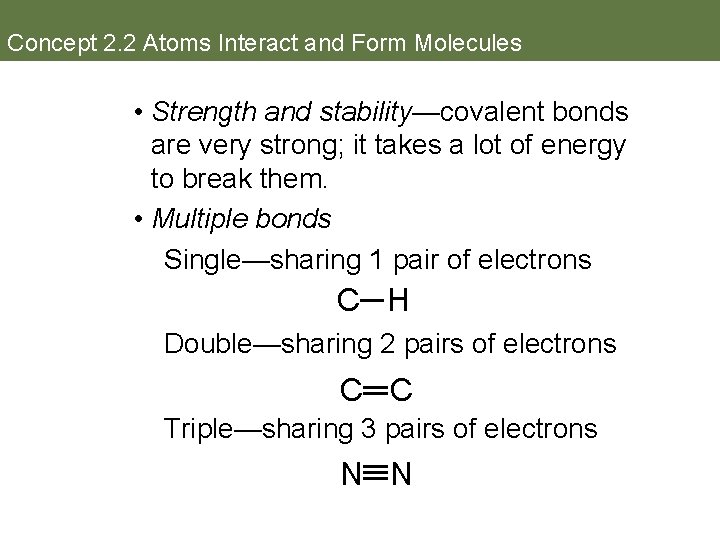
Concept 2. 2 Atoms Interact and Form Molecules • Strength and stability—covalent bonds are very strong; it takes a lot of energy to break them. • Multiple bonds Single—sharing 1 pair of electrons C H Double—sharing 2 pairs of electrons C C Triple—sharing 3 pairs of electrons N N

Concept 2. 2 Atoms Interact and Form Molecules Two atoms of different elements do not always share electrons equally. The nucleus of one element may have greater electronegativity—the attractive force that an atomic nucleus exerts on electrons. Depends on the number of protons and the distance between the nucleus and electrons.

Concept 2. 2 Atoms Interact and Form Molecules If atoms have similar electronegativities, they share electrons equally (nonpolar covalent bond). If atoms have different electronegativities, electrons tend to be near the most attractive atom, forming a polar covalent bond.

Concept 2. 2 Atoms Interact and Form Molecules The partial charges that result from polar covalent bonds produce polar molecules or polar regions of large molecules. Polar bonds influence interactions with other molecules. Polarity of water molecules determines many of water’s unique properties.

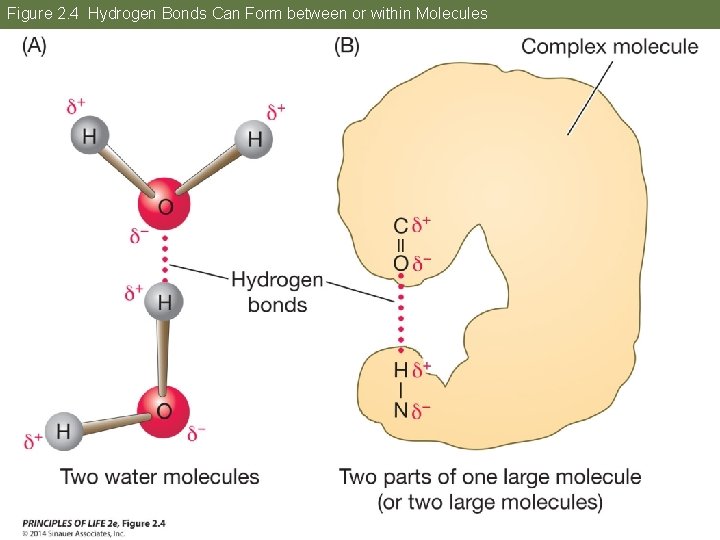
Concept 2. 2 Atoms Interact and Form Molecules Hydrogen bonds: Attraction between the δ– end of one molecule and the δ+ hydrogen end of another molecule. They form between water molecules and within larger molecules. Although much weaker than covalent bonds, they are important in the structure of DNA and proteins.

Figure 2. 4 Hydrogen Bonds Can Form between or within Molecules

Concept 2. 2 Atoms Interact and Form Molecules Hydrogen bonding contributes to properties of water that are significant for life: • Water is a solvent in living systems—a liquid in which other molecules dissolve. • Water molecules form multiple hydrogen bonds with each other—this contributes to high heat capacity.

Concept 2. 2 Atoms Interact and Form Molecules A lot of heat energy is required to raise the temperature of water—the heat energy breaks the hydrogen bonds. In organisms, presence of water shields them from fluctuations in environmental temperature.

Concept 2. 2 Atoms Interact and Form Molecules • Water has a high heat of vaporization: a lot of heat energy is required to change water from the liquid to gaseous state (to break the hydrogen bonds). § Thus, evaporation has a cooling effect on the environment. § Sweating cools the body—as sweat evaporates from the skin, it absorbs some of the adjacent body heat.

Concept 2. 2 Atoms Interact and Form Molecules • Hydrogen bonds give water cohesive strength, or cohesion—water molecules resist coming apart when placed under tension. • Hydrogen bonding between liquid water molecules and solid surfaces allows for adhesion between the water and the solid surface.

In-Text Art, Chapter 2, p. 24

Concept 2. 2 Atoms Interact and Form Molecules § Cohesion and adhesion allow narrow columns of water to move from roots to the leaves of plants. • Surface tension: water molecules at the surface are hydrogen-bonded to other molecules below them, making the surface difficult to puncture. This allows spiders to walk on the surface of a pond.

Concept 2. 2 Atoms Interact and Form Molecules Any polar molecule can interact with any other polar molecule through hydrogen bonds. • Hydrophilic (“water-loving”): in aqueous solutions, polar molecules become separated and surrounded by water molecules. Nonpolar molecules are called hydrophobic (“water-hating”); the interactions between them are hydrophobic interactions.

Concept 2. 2 Atoms Interact and Form Molecules Cations—positively charged ions Anions—negatively charged ions Ionic attractions result from the electrical attraction between ions with opposite charges. The resulting molecules are called salts or ionic compounds.

Concept 2. 2 Atoms Interact and Form Molecules Functional groups—small groups of atoms with specific chemical properties Functional groups confer these properties to larger molecules (e. g. , polarity). One biological molecule may contain many functional groups that determine molecular shape and reactivity.

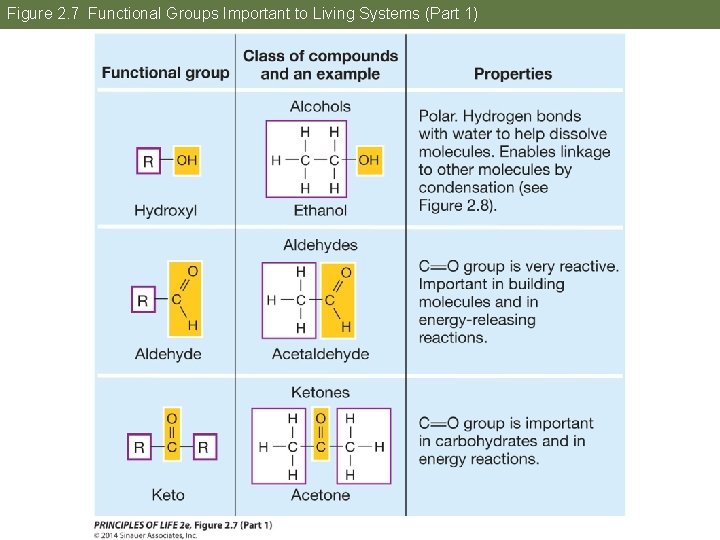
Figure 2. 7 Functional Groups Important to Living Systems (Part 1)

Figure 2. 7 Functional Groups Important to Living Systems (Part 2)

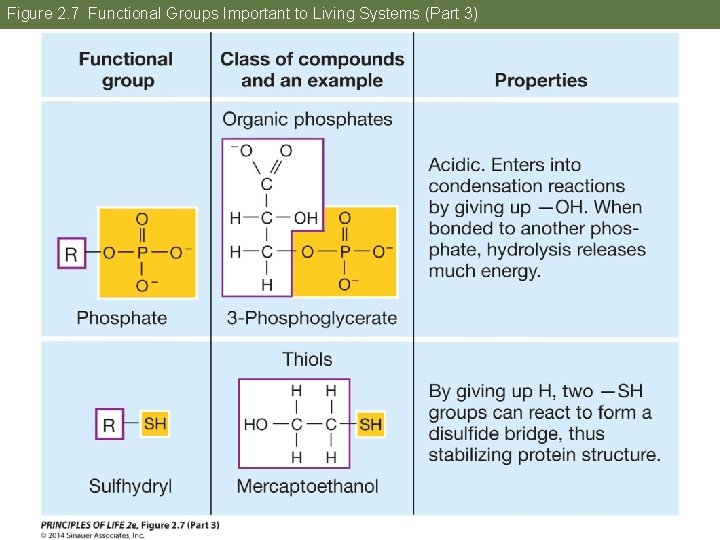
Figure 2. 7 Functional Groups Important to Living Systems (Part 3)

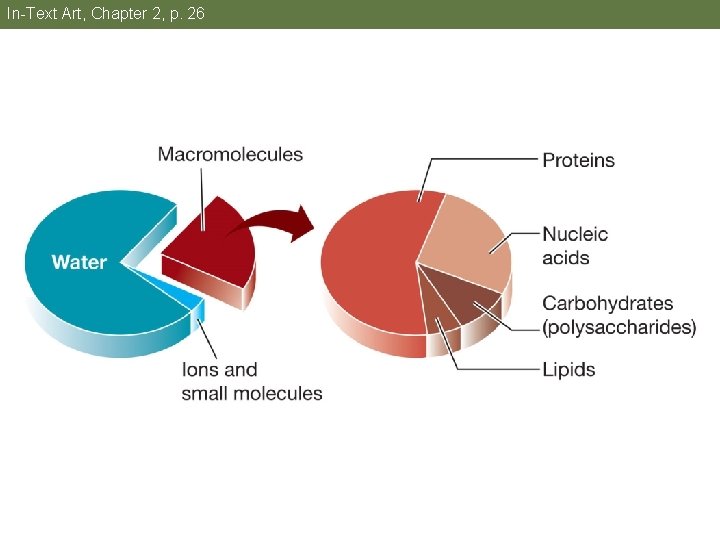
In-Text Art, Chapter 2, p. 26

Biomolecules • Carbohydrates, lipids, proteins, and nucleic acids are called biomolecules. § Usually consist of many repeating units • Each repeating unit is called a monomer. • A molecule composed of monomers is called a polymer (many parts). – Example: amino acids (monomer) are joined together to form a protein (polymer)

Concept 2. 2 Atoms Interact and Form Molecules • Proteins—formed from different combinations of 20 amino acids • Carbohydrates—formed by linking sugar monomers (monosaccharides) to form polysaccharides • Nucleic acids—formed from four kinds of nucleotide monomers • Lipids—noncovalent forces maintain the interactions between the lipid monomers

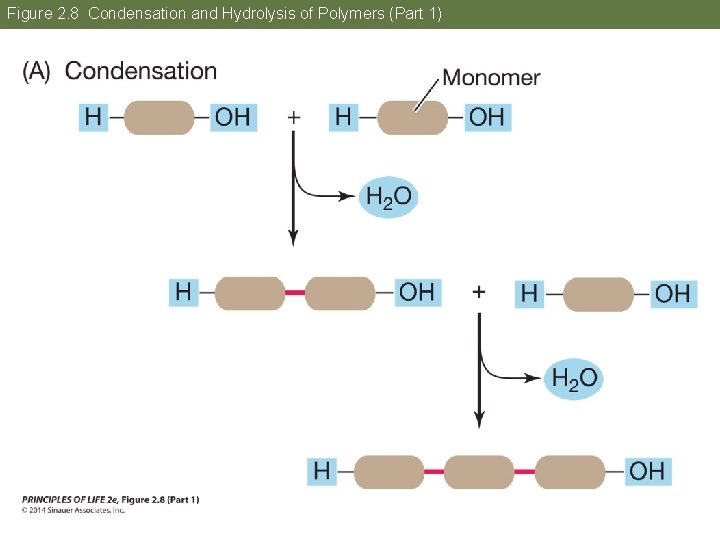
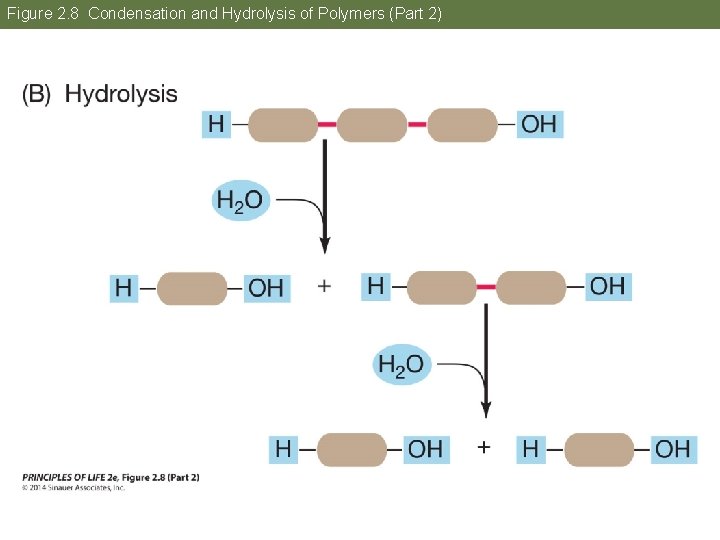
Concept 2. 2 Atoms Interact and Form Molecules Polymers are formed and broken apart in reactions involving water. • Condensation—removal of water links monomers together • Hydrolysis—addition of water breaks a polymer into monomers

Figure 2. 8 Condensation and Hydrolysis of Polymers (Part 1)

Figure 2. 8 Condensation and Hydrolysis of Polymers (Part 2)

Concept 2. 3 Carbohydrates Consist of Sugar Molecules Carbohydrates • Source of stored energy • Transport stored energy within organisms • Structural molecules give many organisms their shapes • Recognition or signaling molecules can trigger specific biological responses

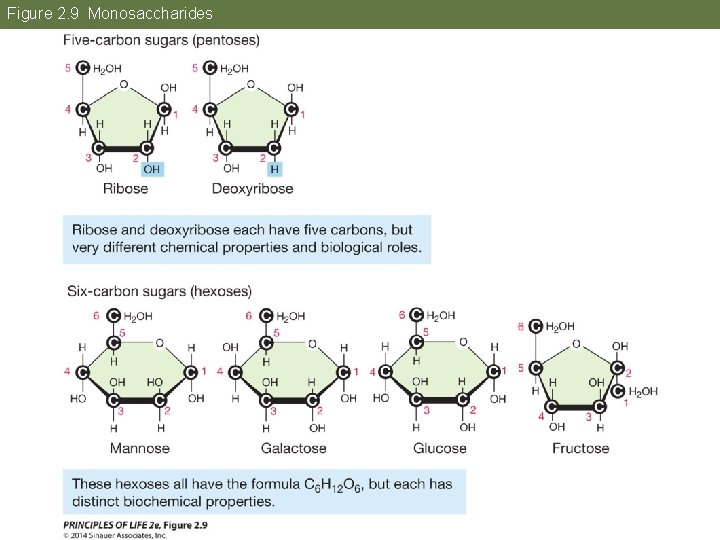
Concept 2. 3 Carbohydrates Consist of Sugar Molecules Monosaccharides are simple sugars. Pentoses are 5 -carbon sugars. • Ribose and deoxyribose are the backbones of RNA and DNA. Hexoses (C 6 H 12 O 6) include glucose, fructose, mannose, and galactose.

Figure 2. 9 Monosaccharides

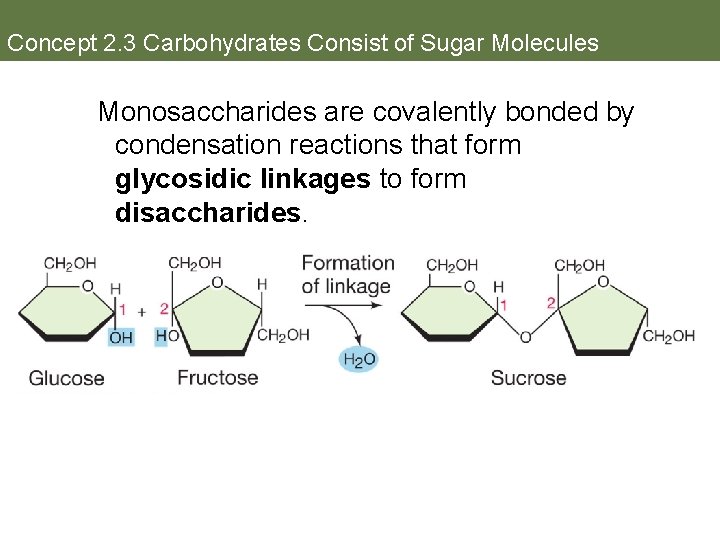
Concept 2. 3 Carbohydrates Consist of Sugar Molecules Monosaccharides are covalently bonded by condensation reactions that form glycosidic linkages to form disaccharides. place text art pg 27 here

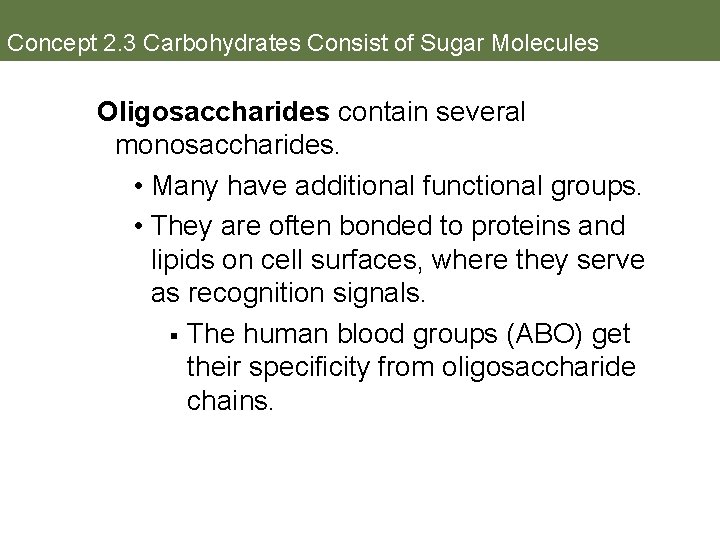
Concept 2. 3 Carbohydrates Consist of Sugar Molecules Oligosaccharides contain several monosaccharides. • Many have additional functional groups. • They are often bonded to proteins and lipids on cell surfaces, where they serve as recognition signals. § The human blood groups (ABO) get their specificity from oligosaccharide chains.

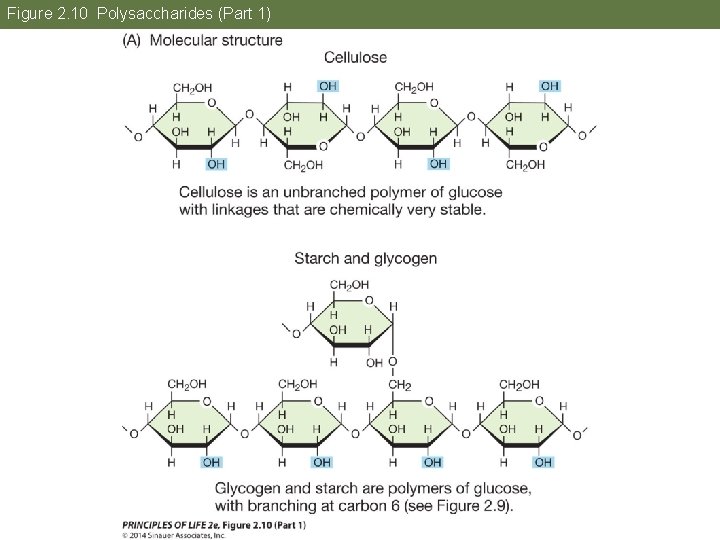
Concept 2. 3 Carbohydrates Consist of Sugar Molecules Polysaccharides are large polymers; the chains can be branching. • Starches—polymers of glucose • Glycogen—highly branched polymer of glucose; main energy storage molecule in mammals

Figure 2. 10 Polysaccharides (Part 1)

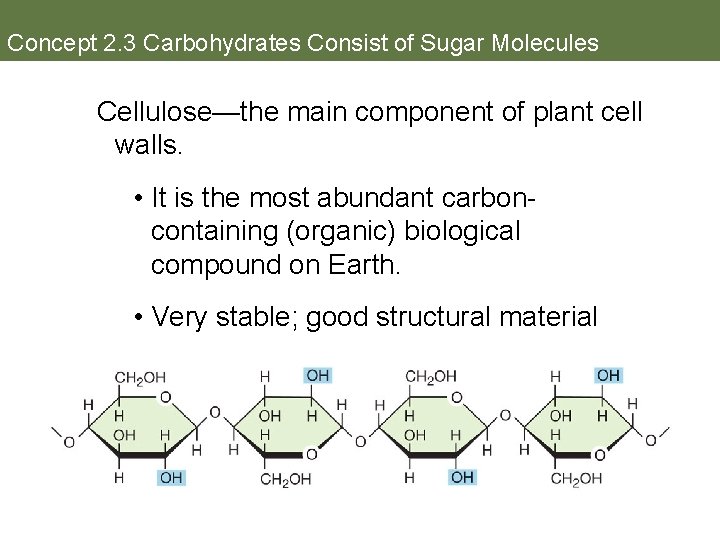
Concept 2. 3 Carbohydrates Consist of Sugar Molecules Cellulose—the main component of plant cell walls. • It is the most abundant carboncontaining (organic) biological compound on Earth. • Very stable; good structural material

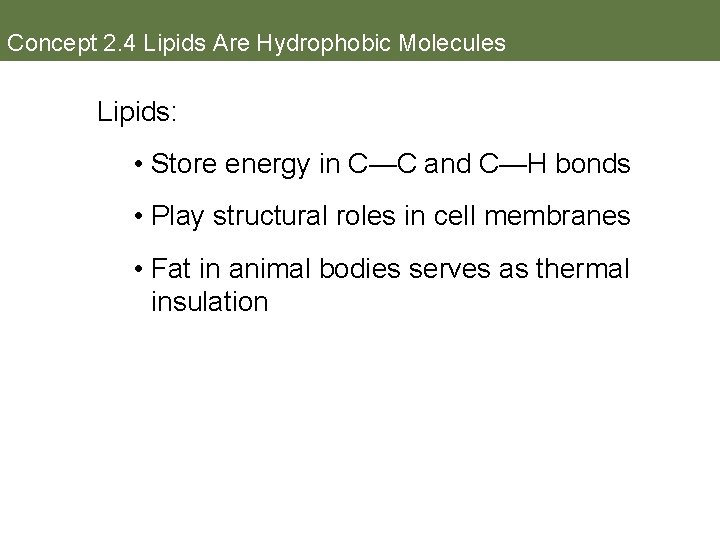
Concept 2. 4 Lipids Are Hydrophobic Molecules Lipids Hydrocarbons (composed of C and H atoms) that are insoluble in water because of many nonpolar covalent bonds. When close together, weak but additive van der Waals interactions hold them together.

Concept 2. 4 Lipids Are Hydrophobic Molecules Lipids: • Store energy in C—C and C—H bonds • Play structural roles in cell membranes • Fat in animal bodies serves as thermal insulation

Concept 2. 4 Lipids Are Hydrophobic Molecules Triglycerides (simple lipids) • Fats—solid at room temperature • Oils—liquid at room temperature • Have very little polarity and are extremely hydrophobic.

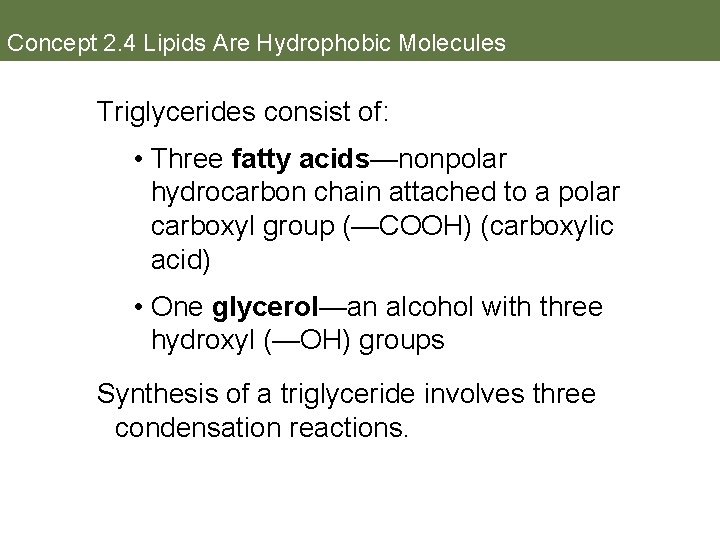
Concept 2. 4 Lipids Are Hydrophobic Molecules Triglycerides consist of: • Three fatty acids—nonpolar hydrocarbon chain attached to a polar carboxyl group (—COOH) (carboxylic acid) • One glycerol—an alcohol with three hydroxyl (—OH) groups Synthesis of a triglyceride involves three condensation reactions.

Figure 2. 11 Synthesis of a Triglyceride

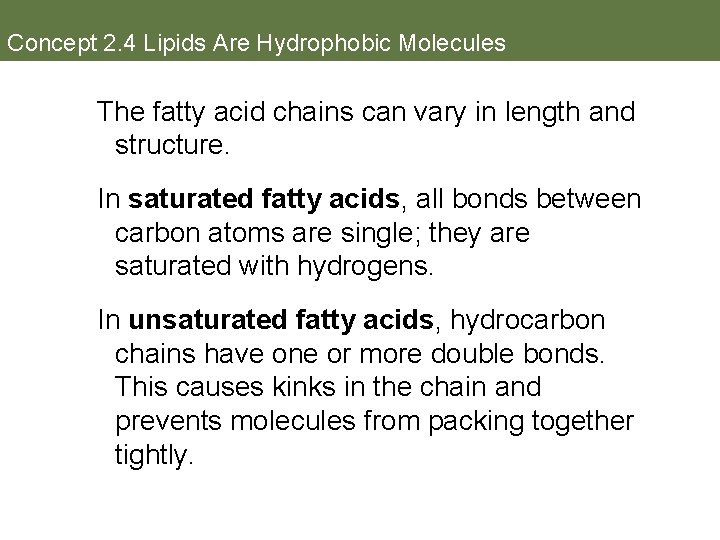
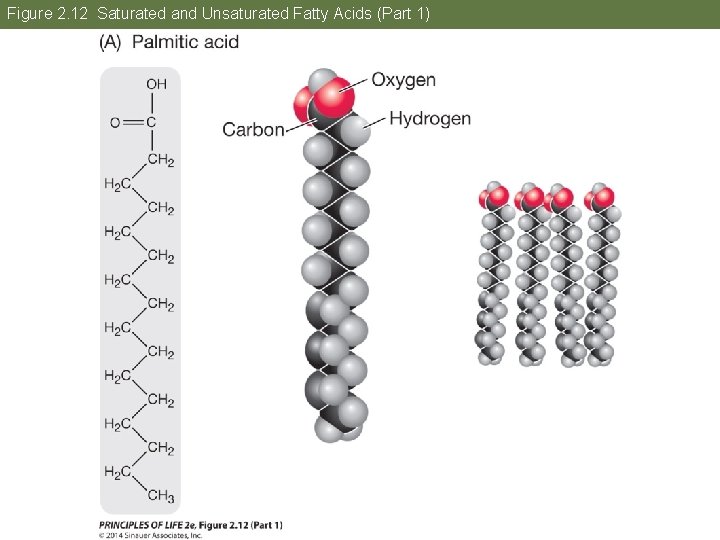
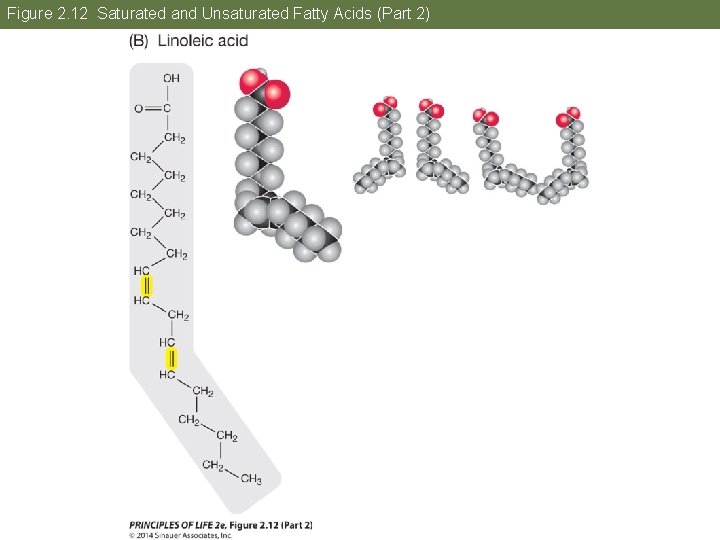
Concept 2. 4 Lipids Are Hydrophobic Molecules The fatty acid chains can vary in length and structure. In saturated fatty acids, all bonds between carbon atoms are single; they are saturated with hydrogens. In unsaturated fatty acids, hydrocarbon chains have one or more double bonds. This causes kinks in the chain and prevents molecules from packing together tightly.

Concept 2. 4 Lipids Are Hydrophobic Molecules Because the unsaturated fatty acids do not pack tightly, they have low melting points and are usually liquid at room temperature. place text art pg 30 here

Figure 2. 12 Saturated and Unsaturated Fatty Acids (Part 1)

Figure 2. 12 Saturated and Unsaturated Fatty Acids (Part 2)

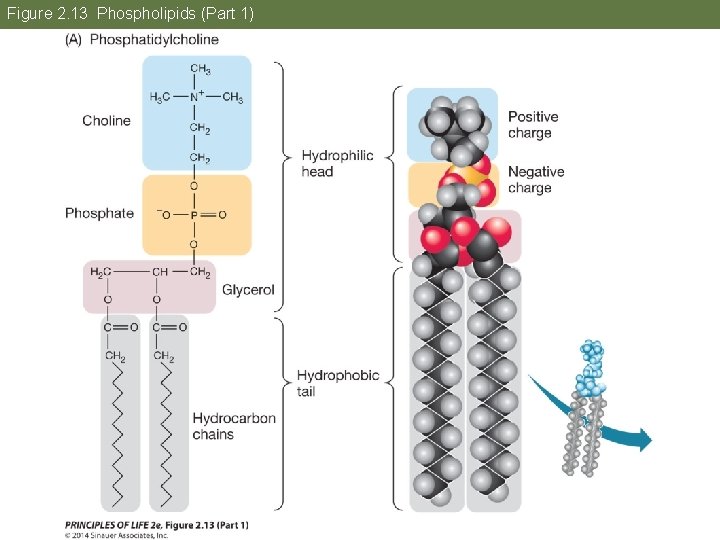
Concept 2. 4 Lipids Are Hydrophobic Molecules Fatty acids are amphipathic; they have a hydrophilic end a hydrophobic tail. Phospholipid—two fatty acids and a phosphate group bound to glycerol; The phosphate group has a negative charge, making that part of the molecule hydrophilic.

Figure 2. 13 Phospholipids (Part 1)

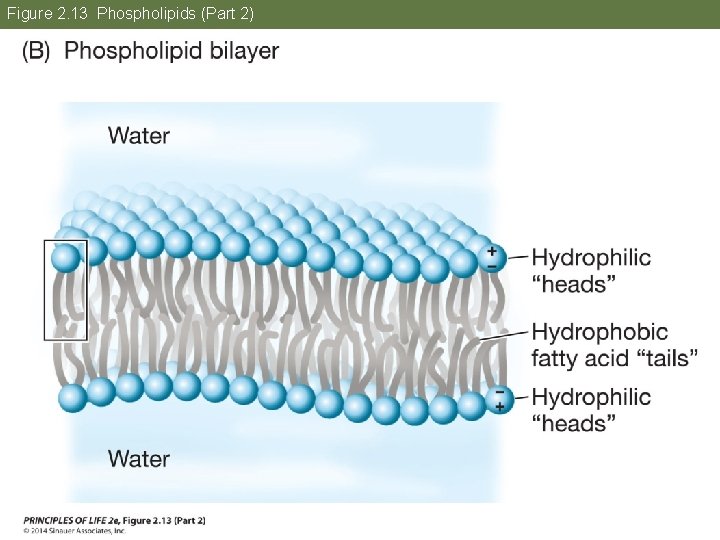
Concept 2. 4 Lipids Are Hydrophobic Molecules In an aqueous environment, phospholipids form a bilayer. The nonpolar, hydrophobic “tails” pack together and the phosphate-containing “heads” face outward, where they interact with water. Biological membranes have this kind of phospholipid bilayer structure.

Figure 2. 13 Phospholipids (Part 2)

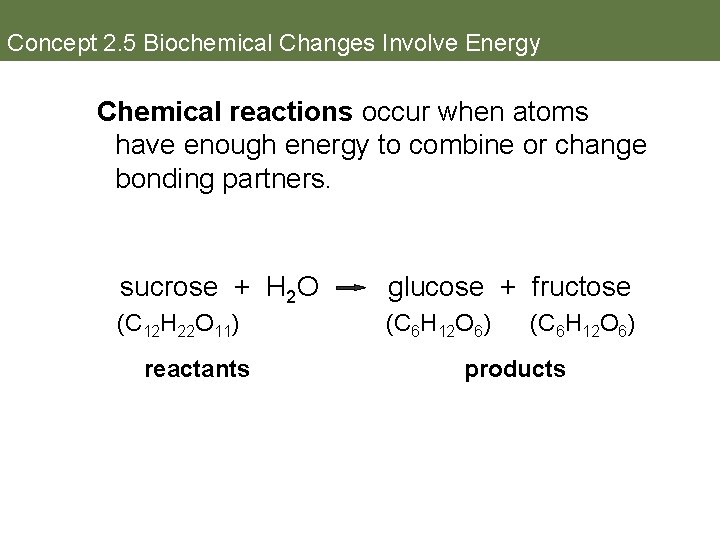
Concept 2. 5 Biochemical Changes Involve Energy Chemical reactions occur when atoms have enough energy to combine or change bonding partners. sucrose + H 2 O glucose + fructose (C 12 H 22 O 11) (C 6 H 12 O 6) reactants (C 6 H 12 O 6) products

Concept 2. 5 Biochemical Changes Involve Energy Chemical reactions involve changes in energy. Energy can be defined as the capacity to do work, or the capacity for change. In biochemical reactions, energy changes are usually associated with changes in the chemical composition and properties of molecules.

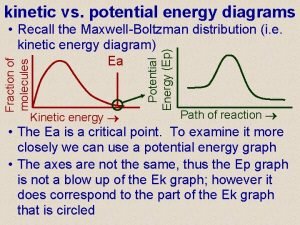
Concept 2. 5 Biochemical Changes Involve Energy All forms of energy can be considered as either: • Potential—the energy of state or position, or stored energy • Kinetic—the energy of movement; the type of energy that does work; that makes things change Energy can be converted from one form to another.

Concept 2. 5 Biochemical Changes Involve Energy Metabolism—sum total of all chemical reactions occurring in a biological system at a given time Metabolic reactions involve energy changes. Energy is either stored in, or released from, chemical bonds. A chemical reaction will occur spontaneously if the total energy consumed by breaking bonds in the reactants is less than the total energy released by forming bonds in the products.

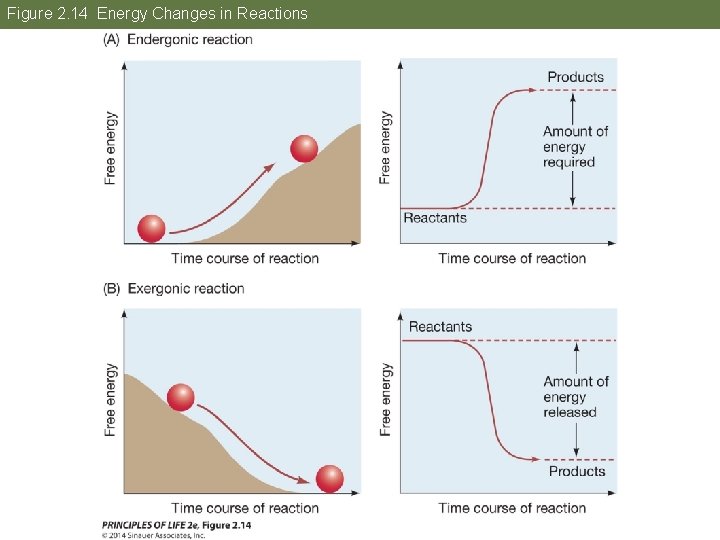
Concept 2. 5 Biochemical Changes Involve Energy Two basic types of metabolism: • Anabolic reactions link simple molecules to form complex ones. § They require energy inputs (endergonic or endothermic; energy is captured in the chemical bonds that form.

Figure 2. 14 Energy Changes in Reactions

Concept 2. 5 Biochemical Changes Involve Energy • Catabolic reactions: energy is released (exergonic or exothermic) § Complex molecules are broken down into simpler ones. § Energy stored in the chemical bonds is released.

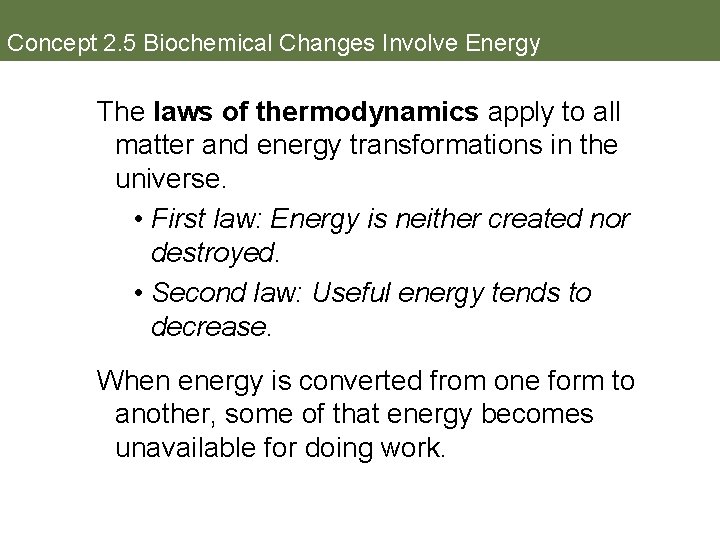
Concept 2. 5 Biochemical Changes Involve Energy Catabolic and anabolic reactions are often linked. The energy released in catabolic reactions is often used to drive anabolic reactions—to do biological work.

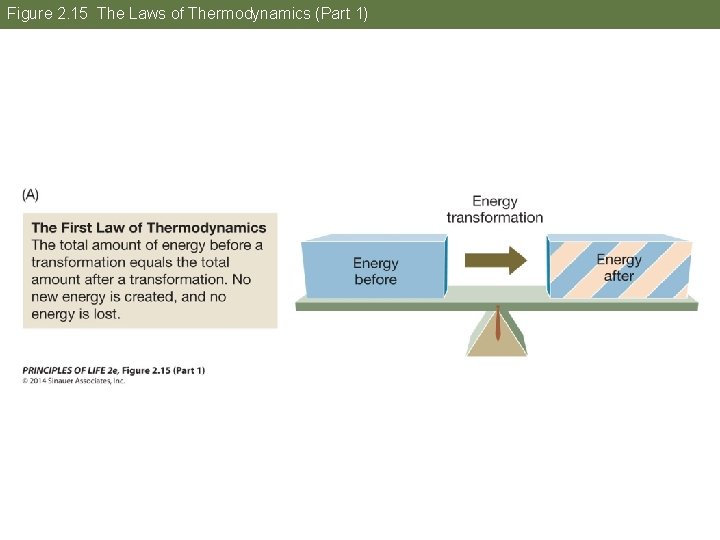
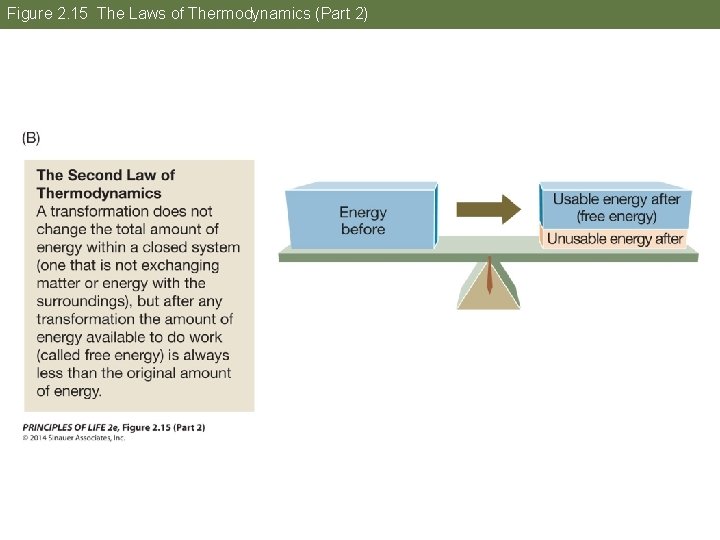
Concept 2. 5 Biochemical Changes Involve Energy The laws of thermodynamics apply to all matter and energy transformations in the universe. • First law: Energy is neither created nor destroyed. • Second law: Useful energy tends to decrease. When energy is converted from one form to another, some of that energy becomes unavailable for doing work.

Figure 2. 15 The Laws of Thermodynamics (Part 1)

Figure 2. 15 The Laws of Thermodynamics (Part 2)

Figure 2. 15 The Laws of Thermodynamics (Part 3)

Concept 2. 5 Biochemical Changes Involve Energy No physical process or chemical reaction is 100% efficient—some of the released energy is lost in a form associated with disorder. This energy is so dispersed that it is unusable. Entropy is a measure of the disorder in a system. As a result of energy transformations, disorder tends to increase.

Concept 2. 5 Biochemical Changes Involve Energy If a chemical reaction increases entropy, its products are more disordered or random than its reactants. If there are fewer products than reactants, the disorder is reduced; this requires energy to achieve.

Concept 2. 5 Biochemical Changes Involve Energy Metabolism creates more disorder (more energy is lost to entropy) than the amount of order that is stored. Example: • The anabolic reactions needed to construct 1 kg of animal body require the catabolism of about 10 kg of food. Life requires a constant input of energy to maintain order.

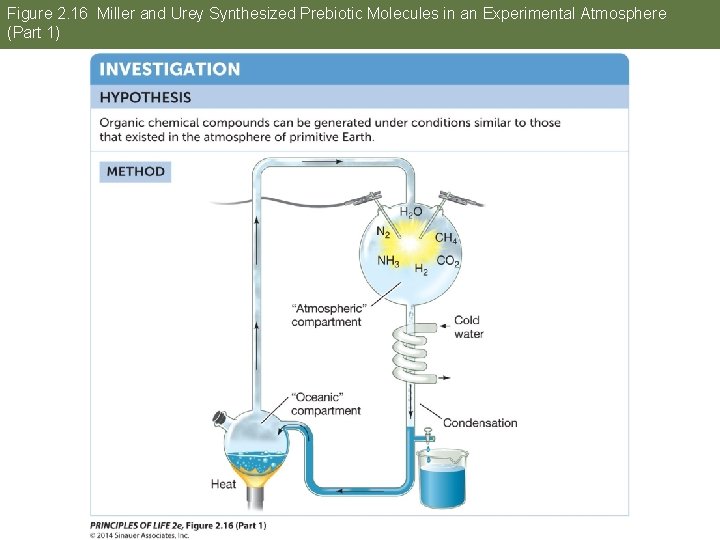
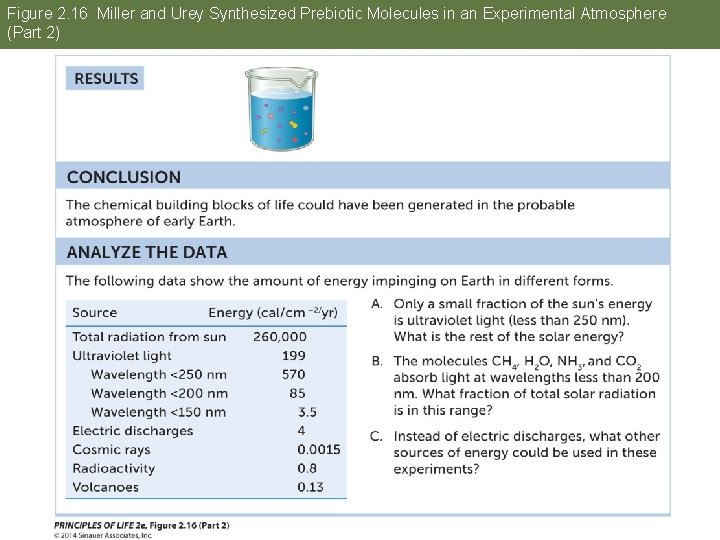
Answer to Opening Question Water is essential for life. One way to investigate the possibility of life on other planets is to study how life may have originated on Earth. Experiments in the 1950 s combined gases thought to be present in Earth’s early atmosphere, including water vapor. An electric spark provided energy. Complex molecules formed, such as amino acids. Water was essential in this experiment.

Figure 2. 16 Miller and Urey Synthesized Prebiotic Molecules in an Experimental Atmosphere (Part 1)

Figure 2. 16 Miller and Urey Synthesized Prebiotic Molecules in an Experimental Atmosphere (Part 2)
 Concept 2 chemistry of life
Concept 2 chemistry of life Concept 2 chemistry of life
Concept 2 chemistry of life Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Ib organic chemistry
Ib organic chemistry Inorganic chemistry vs organic chemistry
Inorganic chemistry vs organic chemistry Ap chemistry entropy and free energy
Ap chemistry entropy and free energy Hình ảnh bộ gõ cơ thể búng tay
Hình ảnh bộ gõ cơ thể búng tay Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Voi kéo gỗ như thế nào
Voi kéo gỗ như thế nào Tư thế worm breton là gì
Tư thế worm breton là gì Alleluia hat len nguoi oi
Alleluia hat len nguoi oi Môn thể thao bắt đầu bằng chữ đua
Môn thể thao bắt đầu bằng chữ đua Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Công của trọng lực
Công của trọng lực Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Mật thư tọa độ 5x5
Mật thư tọa độ 5x5 Làm thế nào để 102-1=99
Làm thế nào để 102-1=99 độ dài liên kết
độ dài liên kết Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Thể thơ truyền thống
Thể thơ truyền thống Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Một số thể thơ truyền thống
Một số thể thơ truyền thống Cái miệng nó xinh thế chỉ nói điều hay thôi
Cái miệng nó xinh thế chỉ nói điều hay thôi Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Biện pháp chống mỏi cơ
Biện pháp chống mỏi cơ đặc điểm cơ thể của người tối cổ
đặc điểm cơ thể của người tối cổ Thế nào là giọng cùng tên? *
Thế nào là giọng cùng tên? * Vẽ hình chiếu đứng bằng cạnh của vật thể
Vẽ hình chiếu đứng bằng cạnh của vật thể Fecboak
Fecboak Thẻ vin
Thẻ vin đại từ thay thế
đại từ thay thế điện thế nghỉ
điện thế nghỉ Tư thế ngồi viết
Tư thế ngồi viết Diễn thế sinh thái là
Diễn thế sinh thái là Dot
Dot So nguyen to
So nguyen to Tư thế ngồi viết
Tư thế ngồi viết Lời thề hippocrates
Lời thề hippocrates Thiếu nhi thế giới liên hoan
Thiếu nhi thế giới liên hoan ưu thế lai là gì
ưu thế lai là gì Sự nuôi và dạy con của hổ
Sự nuôi và dạy con của hổ Khi nào hổ con có thể sống độc lập
Khi nào hổ con có thể sống độc lập Sơ đồ cơ thể người
Sơ đồ cơ thể người Từ ngữ thể hiện lòng nhân hậu
Từ ngữ thể hiện lòng nhân hậu Thế nào là mạng điện lắp đặt kiểu nổi
Thế nào là mạng điện lắp đặt kiểu nổi States of matter concept map
States of matter concept map What is real self means
What is real self means Pengertian marketing concept
Pengertian marketing concept Binding energy chemistry
Binding energy chemistry Kinetic energy
Kinetic energy What is lattice energy
What is lattice energy E=mc2 cda
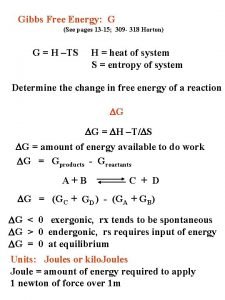
E=mc2 cda Gibbs free
Gibbs free You light up my life answer key
You light up my life answer key Kinetics half life
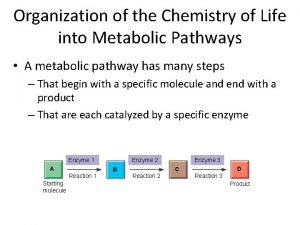
Kinetics half life Chemistry in biology section 4 the building blocks of life
Chemistry in biology section 4 the building blocks of life Chapter 2 the chemistry of life section review 2-2
Chapter 2 the chemistry of life section review 2-2 Deficiency disease of protein
Deficiency disease of protein How is thermal energy generated
How is thermal energy generated Chemistry for life
Chemistry for life Chemical potential energy images
Chemical potential energy images Primary energy and secondary energy
Primary energy and secondary energy What is commercial energy source
What is commercial energy source Helmholtz free energy and gibbs free energy
Helmholtz free energy and gibbs free energy Renewable energy and energy efficiency partnership
Renewable energy and energy efficiency partnership Potential enrgy
Potential enrgy You and your friend both solve a problem involving a skier
You and your friend both solve a problem involving a skier