Nuclear Chemistry Inorganic Chemistry May 12 2015 Atomic








- Slides: 9

Nuclear Chemistry Inorganic Chemistry May 12, 2015

Atomic Nuclei and Nuclear Stability Describe how the strong force attracts nucleons Relate binding energy and mass defect Predict the stability of a nucleus by considering factors such as nuclear size, binding energy, and the ratio of neutrons to protons in the nucleus

Nuclear forces Nucleons and nuclide

Nuclear forces Protons and neutrons are nucleons A specific number of protons and neutrons are described as a nuclide An isotope has the same atomic number but different mass number Isotopic notation Mass # Atomic # 25 12 Mg. Chemical Symbol

Nuclear Forces Strong force exists when nucleons are close together The strong force must overcome the electrical charge of the protons Three quarks make up nucleons Quarks are in flavors

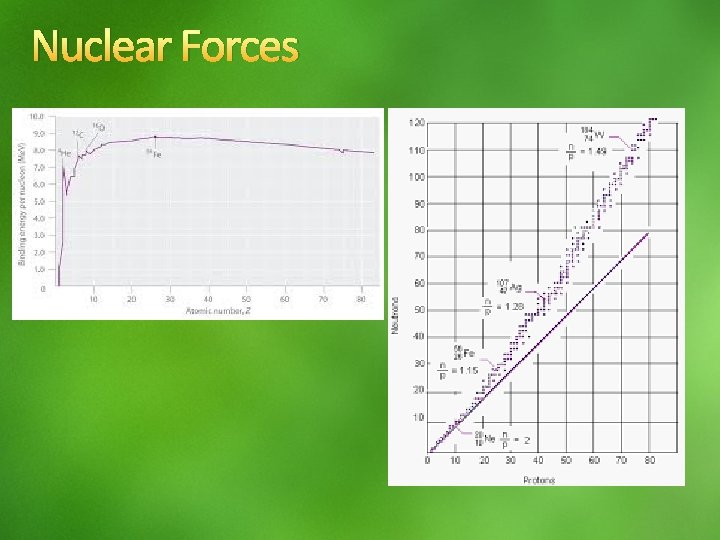
Nuclear Forces Binding energy and Mass Defect separate nucleons nucleus + energy Nuclear binding energy is the energy associated with the loss of mass, mass defect, from the combining of nucleons Eo = mc 2 4 He 2. 73 x 10 12 J/mol 2

Nuclear Forces

Nuclear Forces Stability 1. Except for 11 H and 32 He, all stable nuclei have a number of neutrons that is equal to or greater than the number of protons A nucleus that has an N/Z number that is too large or too small is unstable. Gradual increase from 1 to 1. 5 Nuclei with even number of neutrons and protons are more stable Nuclei that have magic numbers of protons and neutrons [2, 8, 20, 28, 82, &126]

Review Problems 1 -12 Skip 2’s page 647 1 -9 Skip 2’s page 874 Mass of a proton 1. 672614 x 10 -27 kg Mass of a neutron 1. 6749543 x 10 -27 kg Speed of light 3. 00 x 108 m/s
 Inorganic vs organic chemistry
Inorganic vs organic chemistry Lesson 15 nuclear quest nuclear reactions
Lesson 15 nuclear quest nuclear reactions Fisión nuclear vs fision nuclear
Fisión nuclear vs fision nuclear Importance of inorganic chemistry in pharmacy
Importance of inorganic chemistry in pharmacy Advanced inorganic chemistry lecture notes
Advanced inorganic chemistry lecture notes Organic vs inorganic
Organic vs inorganic Inert pair effect
Inert pair effect Is inorganic chemistry hard
Is inorganic chemistry hard Relative formula mass of hcl
Relative formula mass of hcl Periodic table tends
Periodic table tends