The ILC Foundation Ehlers Danlos Complex Dermatologic Features



























- Slides: 99

The ILC Foundation: Ehlers Danlos Complex Dermatologic Features and Issues Scott Walsh MD Ph. D FRCPC Sunnybrook Health Sciences Centre University of Toronto Peter Gilgan Centre November 2 and 3, 2019

Disclosures: No conflicts with Industry relevant to this talk.

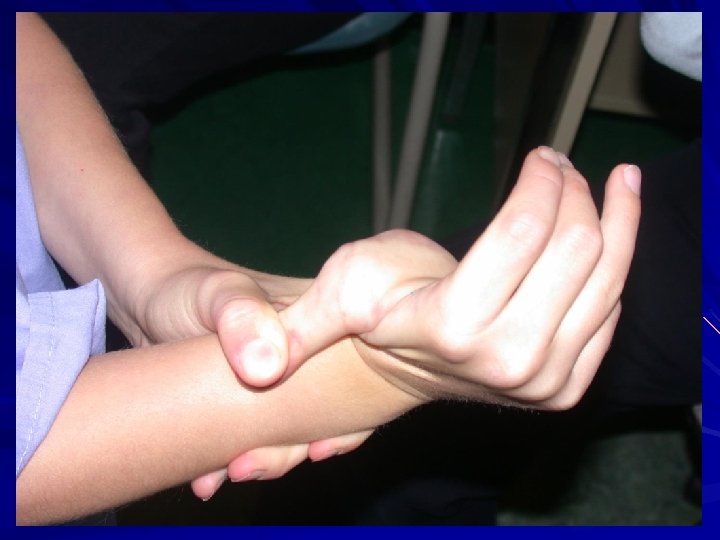
Ehlers Danlos Syndromes: Cardinal features of EDS: Hyper-extensibility of the skin. Hypermobility of the joints. Tissue fragility (skin, blood vessels). Many different types of EDS.

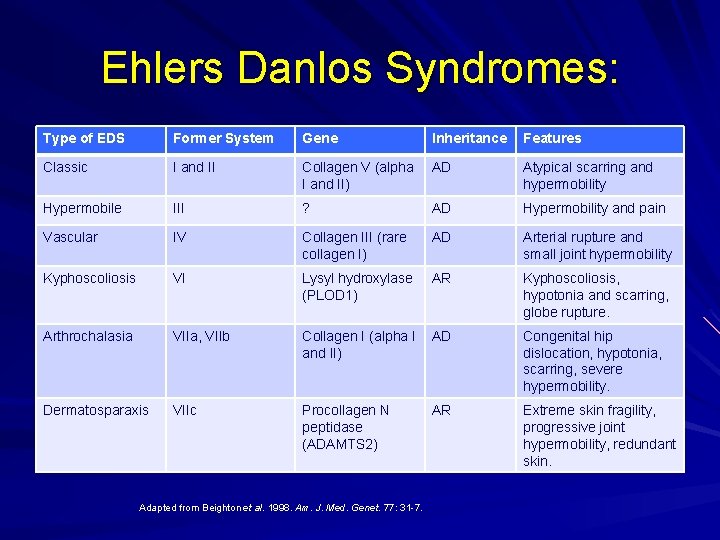
Ehlers Danlos Syndromes: Type of EDS Former System Gene Inheritance Features Classic I and II Collagen V (alpha I and II) AD Atypical scarring and hypermobility Hypermobile III ? AD Hypermobility and pain Vascular IV Collagen III (rare collagen I) AD Arterial rupture and small joint hypermobility Kyphoscoliosis VI Lysyl hydroxylase (PLOD 1) AR Kyphoscoliosis, hypotonia and scarring, globe rupture. Arthrochalasia VIIa, VIIb Collagen I (alpha I and II) AD Congenital hip dislocation, hypotonia, scarring, severe hypermobility. Dermatosparaxis VIIc Procollagen N peptidase (ADAMTS 2) AR Extreme skin fragility, progressive joint hypermobility, redundant skin. Adapted from Beighton et al. 1998. Am. J. Med. Genet. 77: 31 -7.

Ehlers Danlos Syndromes: Byers and Murray, 2012.

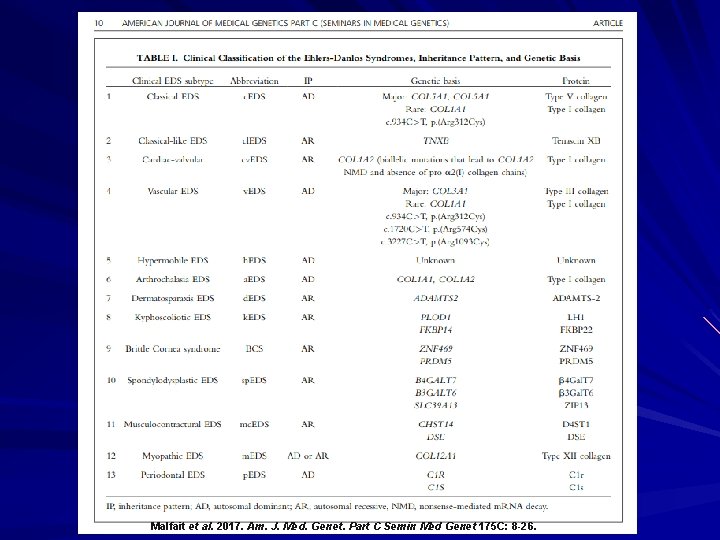
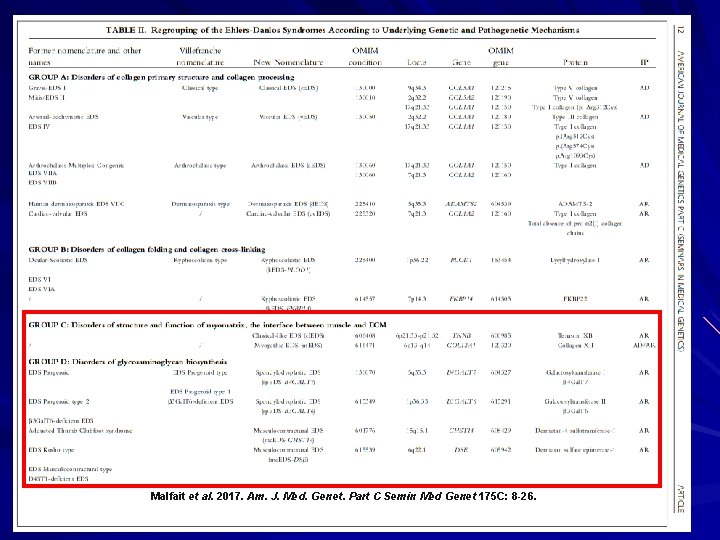
Malfait et al. 2017. Am. J. Med. Genet. Part C Semin Med Genet 175 C: 8 -26.

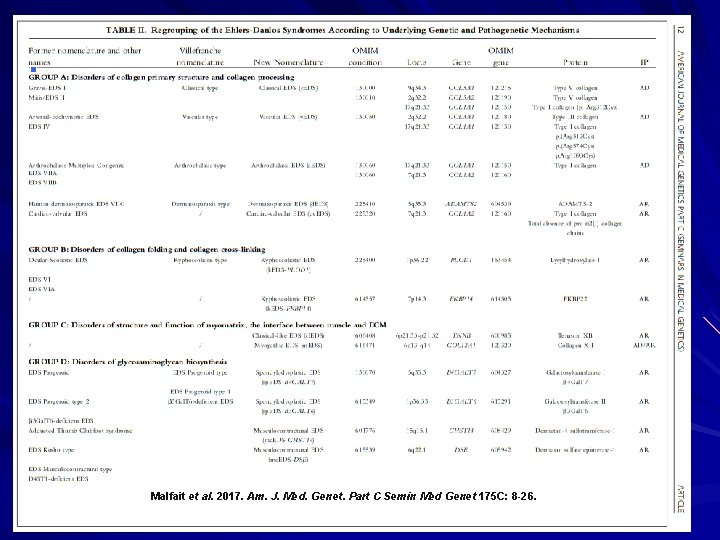
Malfait et al. 2017. Am. J. Med. Genet. Part C Semin Med Genet 175 C: 8 -26.

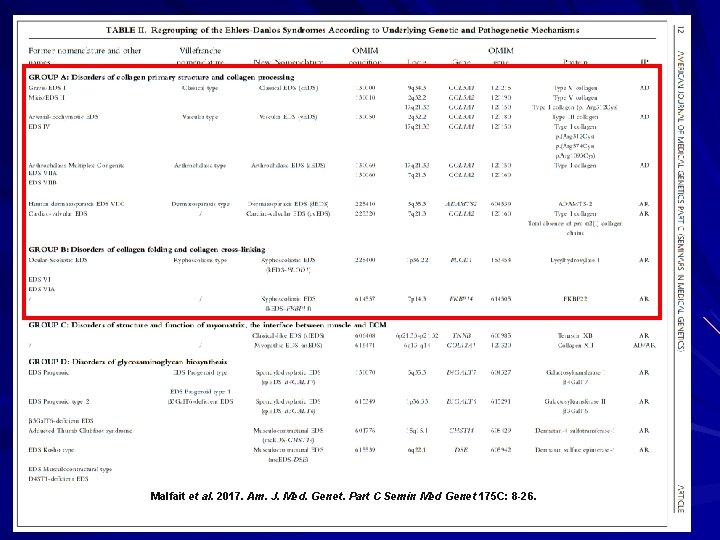
Malfait et al. 2017. Am. J. Med. Genet. Part C Semin Med Genet 175 C: 8 -26.

Malfait et al. 2017. Am. J. Med. Genet. Part C Semin Med Genet 175 C: 8 -26.

Ehlers Danlos Syndromes: Genes affected: – Fibrillar collagens. – Enzymes that process fibrillar collagens. – Proteins that interact with collagens.

Ehlers Danlos Syndromes: Mechanisms: Not enough of the collagen. Haploinsufficiency One bad thread that makes the entire rope bad. Dominant negative effect Collagen that is weaker than normal and falls apart easily. Abnormal solubility and strength

Outline: Review structure of skin with respect to EDS and the interplay of different molecules: Collagen Elastin Ground Substance The role of Electron microscopy in inherited disorders of connective tissue. Hypermobility Spectrum Disorder and changes that can occur with an altered matrix. Effects on activity of other cell types Effects on migration of other cells

Ehlers Danlos Syndromes Skin: Collagen Strength of skin Elastin Resiliency of skin Ground Substance (proteoglycans and glycosaminoglycans) Turgor or texture of skin Abnormality in any one component can affect the proper assembly of the other components.

Ehlers Danlos Syndromes: Disorders primarily of disturbed collagen production (fibrillogenesis). The morphology and strength of collagen is compromised.

Ehlers Danlos Syndromes Collagen is the major protein in skin, ligament, tendon, bone. 29 different types of collagen (43 genes). Triple helix (three threads wrapped around each other to make a tight rope). Homotrimers (proteins from same gene) Heterotrimers (proteins from different genes)

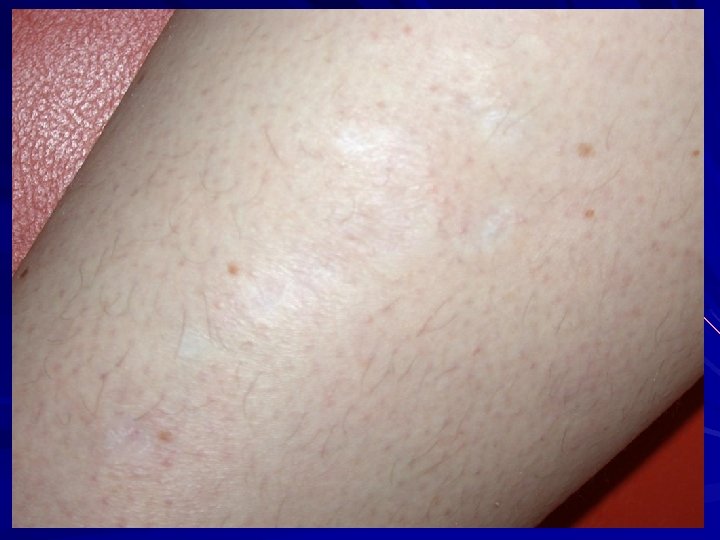
Ehlers Danlos Syndromes "velvety texture" "doughy texture" "thin skin" "hyperextensible" Volar forearm - snap back.

Collagen: Most abundant protein in the body. 70% of dry weight of dermis. Collagen Definition: Have areas where there is a right handed triple-helical domain consisting of 3 left-handed polypeptide chains with a Gly-X-Y sequence where X is often proline and Y is often hydroxyproline. Small amino acids where three threads come together and larger amino acids on the outside of the rope that can interact with other molecules. Structural component of the extracellular matrix.

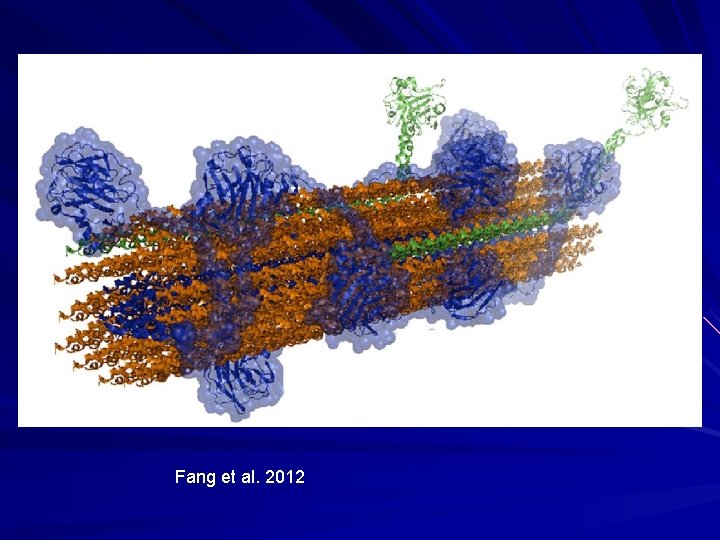
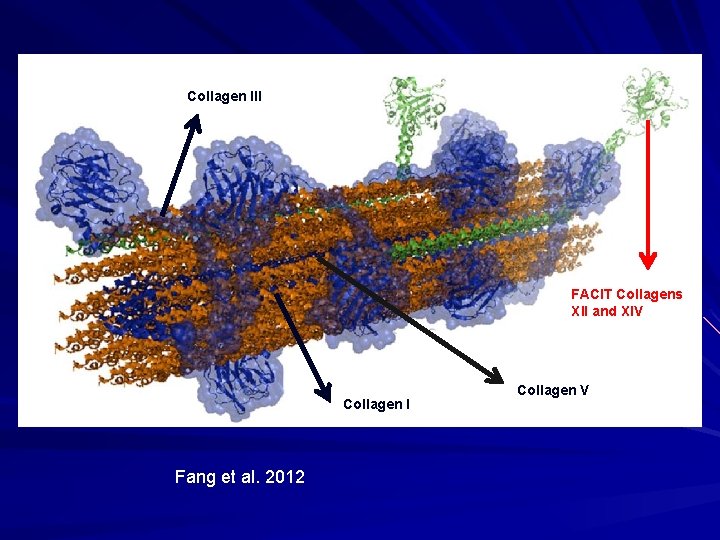
Collagen - Classification Two broad classes: – Fibrillar collagens – form cross-striated fibrils with 67 nm periodicity. – Non-fibrillar collagens – do not form cross-striated fibrils as usually have noncollagenous portions (twists in the rope) that give molecule flexibility (arms for molecules to bind to). – Several of these different types of collagens interact to make a large collagen fibre in the skin.

Fang et al. 2012

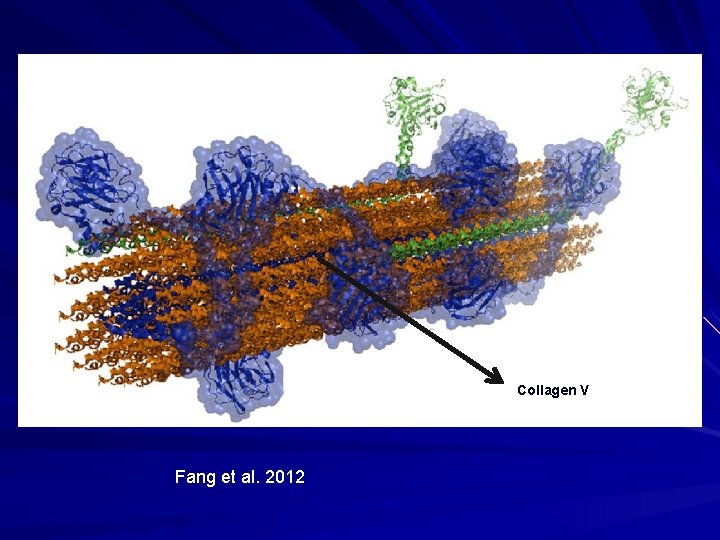
Collagen V Fang et al. 2012

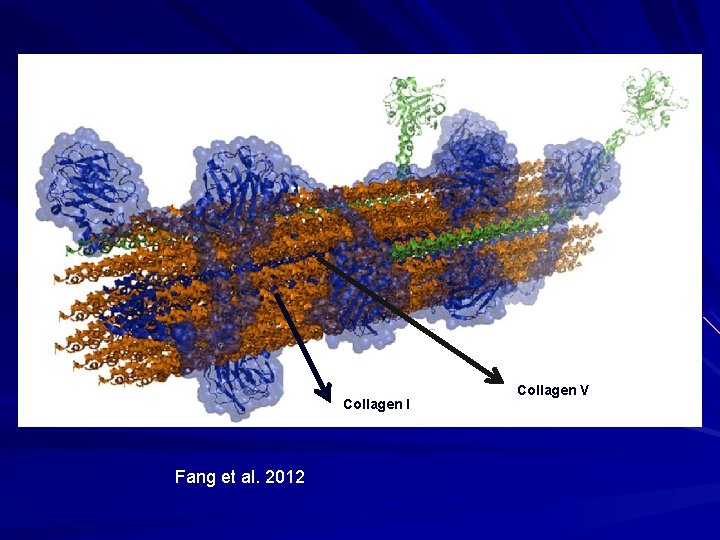
Collagen I Fang et al. 2012 Collagen V

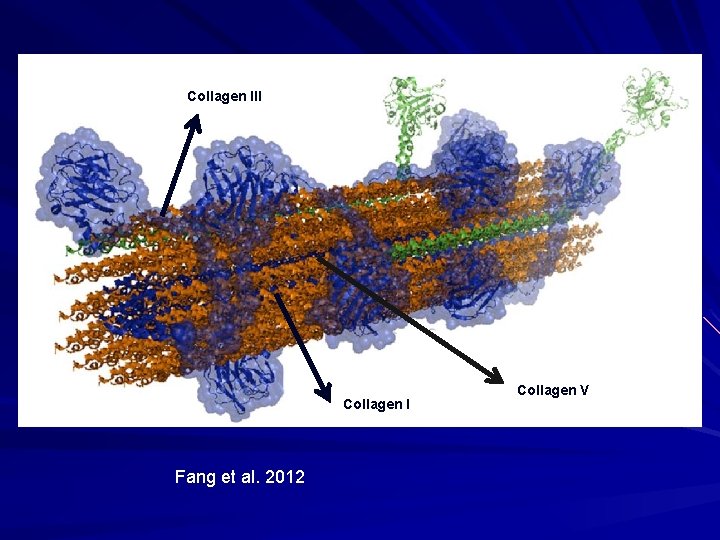
Collagen III Collagen I Fang et al. 2012 Collagen V

Collagen III FACIT Collagens XII and XIV Collagen I Fang et al. 2012 Collagen V

Fibrillar Collagen - V 5% of dermal collagen. Located at core of collagen fibrils in the skin.





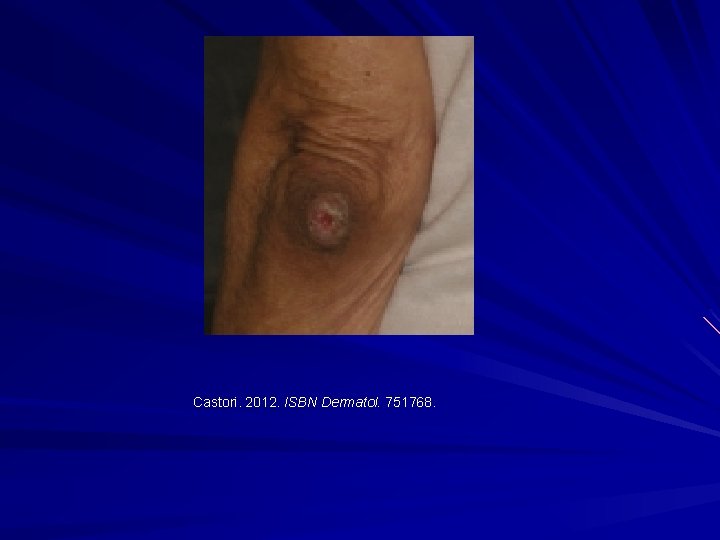
Castori. 2012. ISBN Dermatol. 751768.

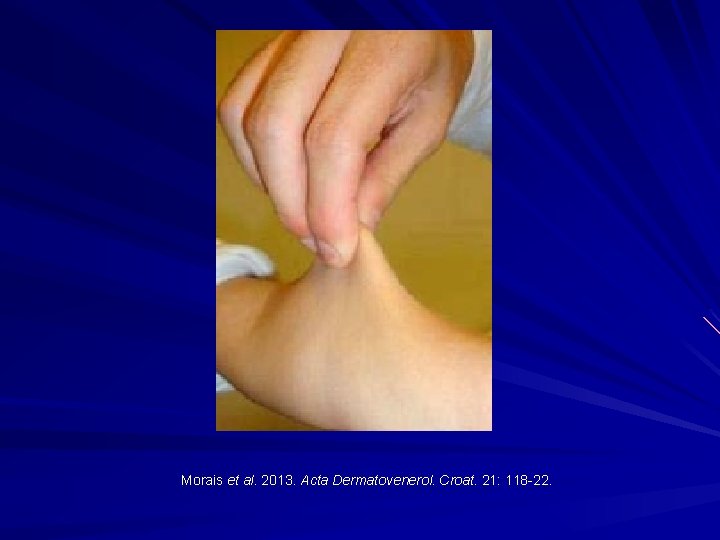
Morais et al. 2013. Acta Dermatovenerol. Croat. 21: 118 -22.

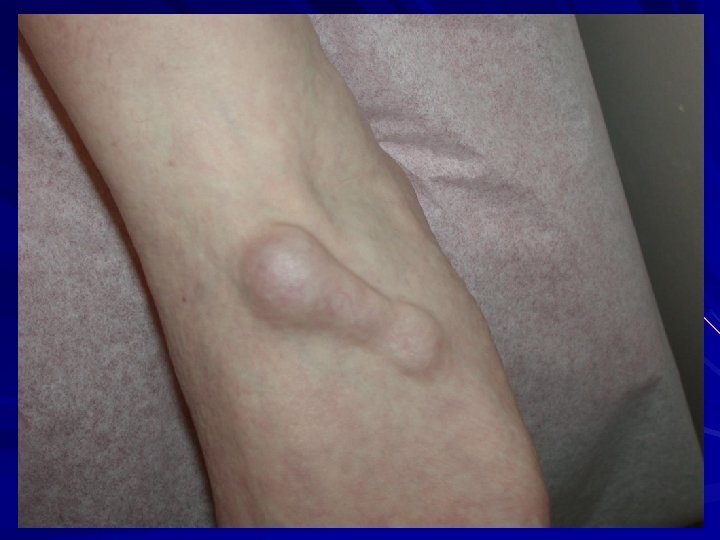
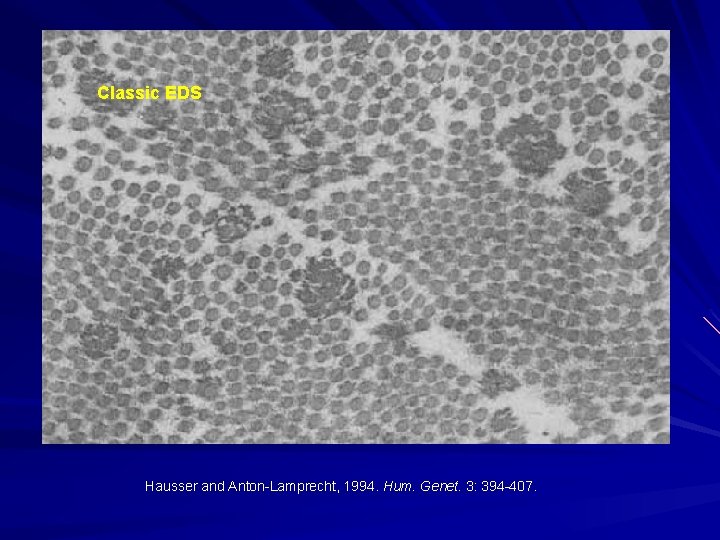
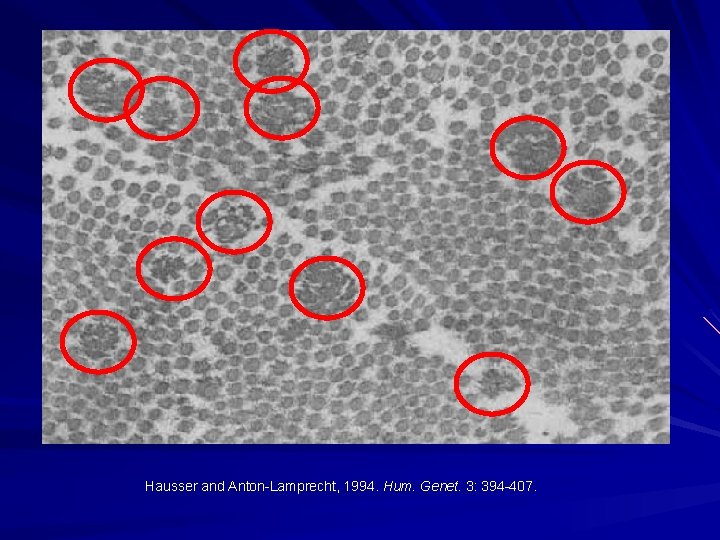
EDS-I/II – Classical type AD Clinical: Thinned/atrophic scars Papyraceus, cigarette paper, fish mouth. Molluscoid pseudotumours, spherules. Hyperextensible skin Joint laxity/hypermobility Small and large joint. COL 5 A 1 or COL 5 A 2 mutations. Morais et al. 2013. Acta. Dermatovenerol. Croat. 21: 118 -22; Malfait et al. 2010. Genet. Med. 12: 597 -605; Malfait et al. 2005. Hum. Mutat. 25: 28 -37.

Fibrillar Collagen - III Embryonic/fetal life. 10% of collagen in adult dermis. Enriched in blood vessels, viscera (GI). Homotrimer; larger than collagen I. Forms a scaffold on which organs and blood vessels are built upon.


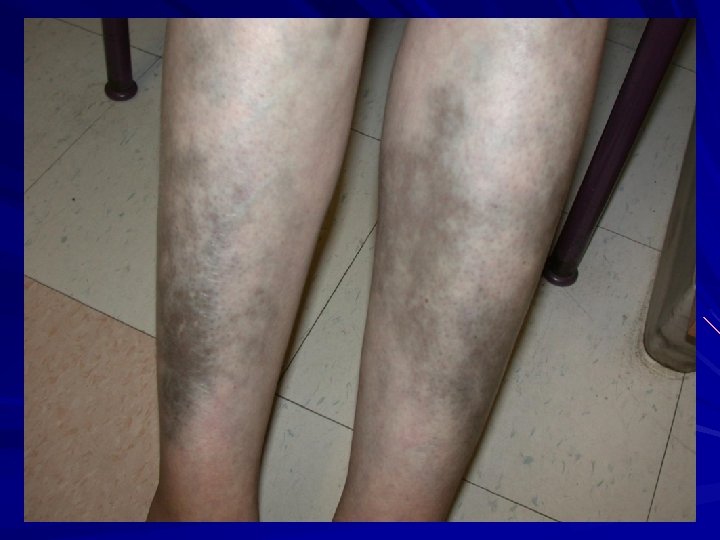
EDS-IV – Vascular/ecchymotic AD with 2 clinical presentations: Acrogeric variety Mutations in triple helix or carboxy-end. Characteristic facies Non-acrogeric Mutations in amino terminus or single null allele. Clinical: Thin, translucent skin Arterial rupture/dissection/aneurysm/bowel perforation/uterine rupture Extensive bruising +/- acrogeria hypermobility of small joints Varicosities family history of sudden death. Rx: ? aortic root graft; avoid NSAIDs, contact sports. 25% one event by 20 years; 80% one event by 40 years. Oderich et al. 2004. J. Vasc. Surg. 42: 98 -106; Germain and Herrera-Guzman, 2004. Ann. Genetic. 47: 1 -9; Germain 2001. Ann. Vasc. Surg. 16: 391 -7

Fibrillar Collagen I Most abundant collagen. 80% of collagen in dermis; bone. Two threads from one gene wrapped with one thread from a second gene. Col 1 A 1 and Col 1 A 2/ Heterotrimer of [ά 1(I)]2ά 2(I). Best studied and model for fibrillar collagen biosynthesis.


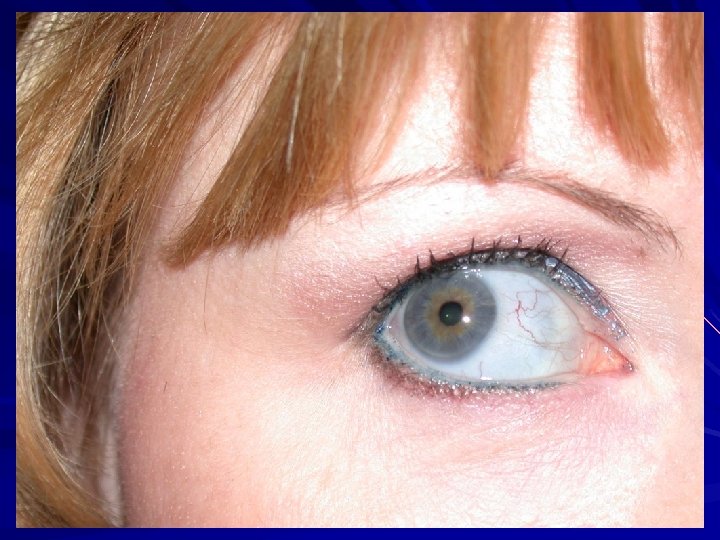
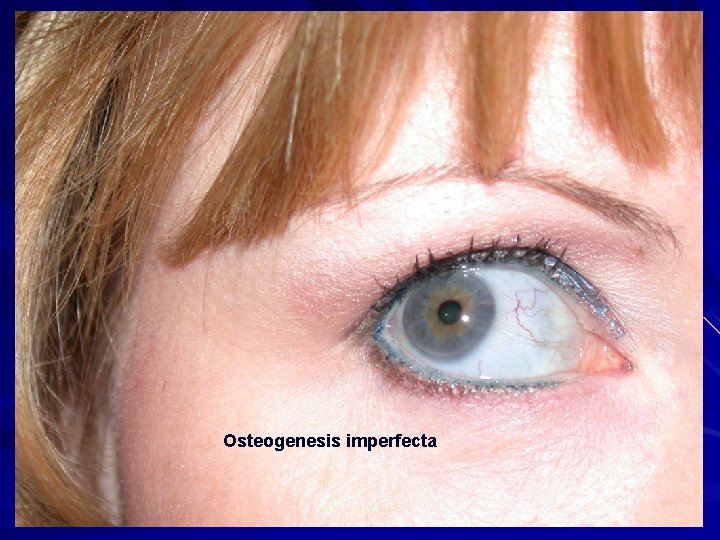
Osteogenesis imperfecta

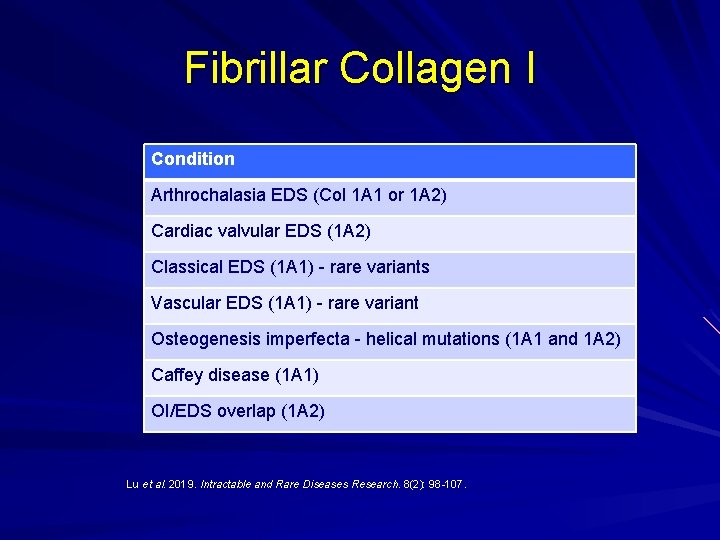
Fibrillar Collagen I Condition Arthrochalasia EDS (Col 1 A 1 or 1 A 2) Cardiac valvular EDS (1 A 2) Classical EDS (1 A 1) - rare variants Vascular EDS (1 A 1) - rare variant Osteogenesis imperfecta - helical mutations (1 A 1 and 1 A 2) Caffey disease (1 A 1) OI/EDS overlap (1 A 2) Lu et al. 2019. Intractable and Rare Diseases Research. 8(2): 98 -107.

Collagen Biosynthesis/Fibrillogenesis: Model Collagen III (fetal) Collagen V (regulatory) Many forms of EDS occur when there is a problem in formation of fibrillar collagen.

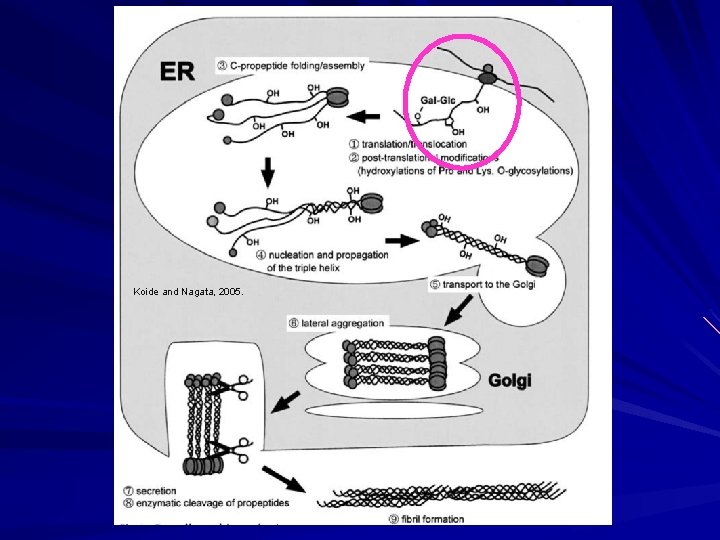
Biosynthesis of Fibrillar Collagen I Formed as a procollagen that becomes an insoluble mature collagen with processing. Transcription: – Upregulated by retinoic acid, TGF-B, insulin, ascorbic acid. – Down-regulated by glucocorticoids, FGF, TNF.

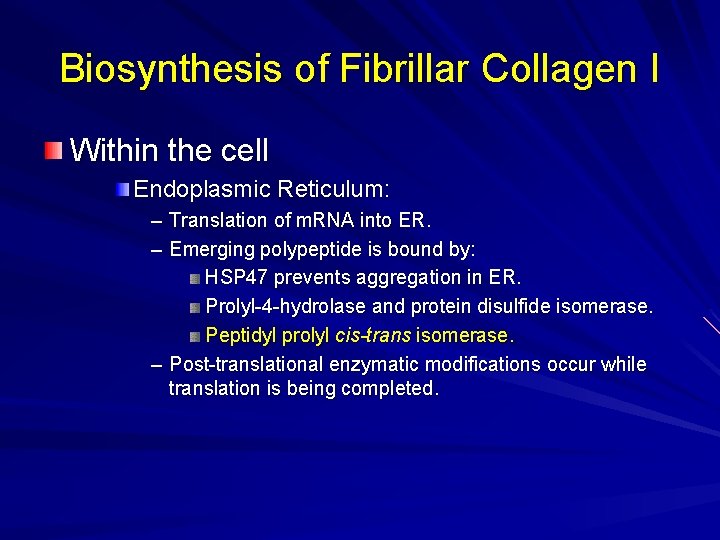
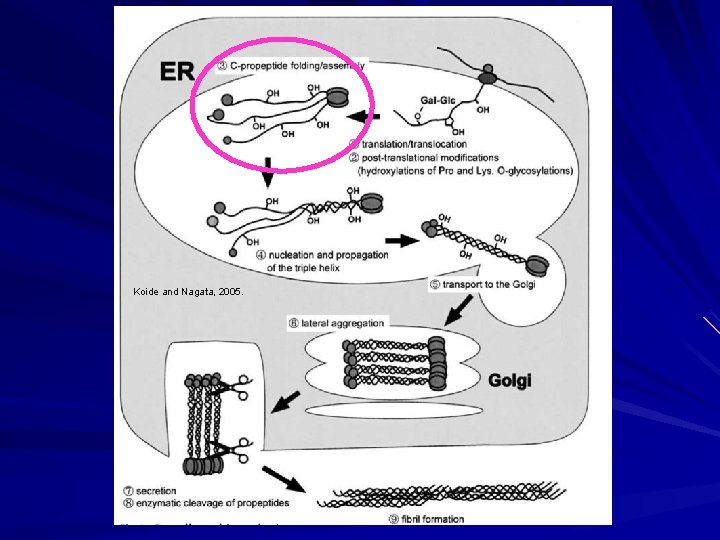
Biosynthesis of Fibrillar Collagen I Within the cell Endoplasmic Reticulum: – Translation of m. RNA into ER. – Emerging polypeptide is bound by: HSP 47 prevents aggregation in ER. Prolyl-4 -hydrolase and protein disulfide isomerase. Peptidyl prolyl cis-trans isomerase. – Post-translational enzymatic modifications occur while translation is being completed.

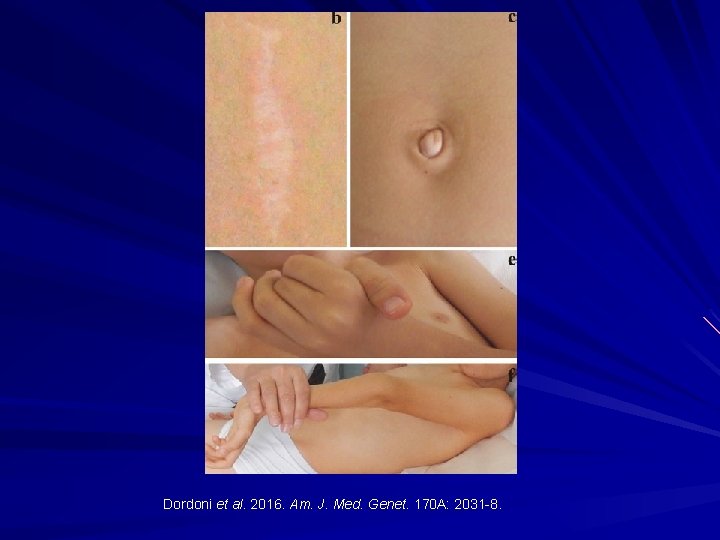
Dordoni et al. 2016. Am. J. Med. Genet. 170 A: 2031 -8.

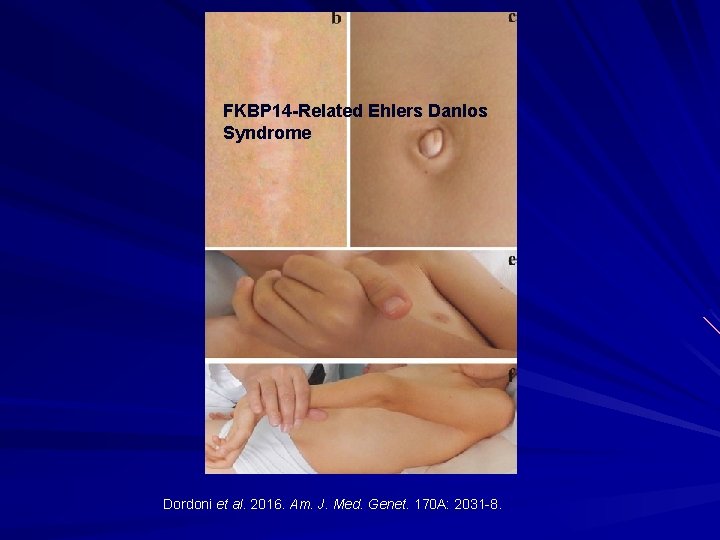
FKBP 14 -Related Ehlers Danlos Syndrome Dordoni et al. 2016. Am. J. Med. Genet. 170 A: 2031 -8.

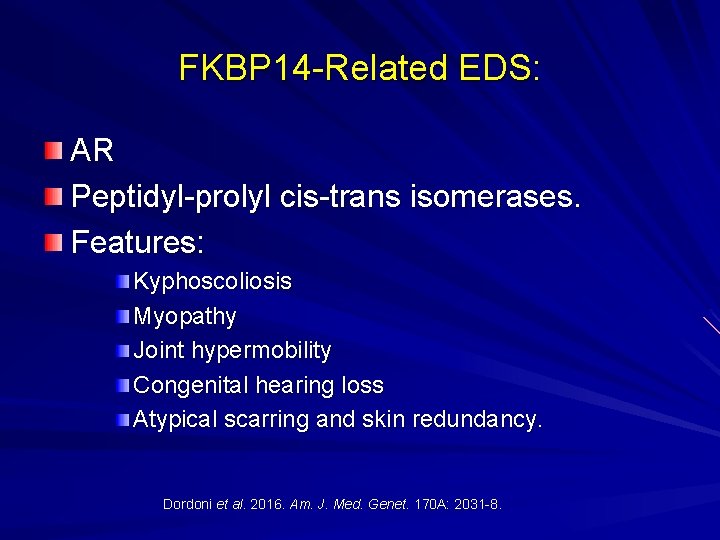
FKBP 14 -Related EDS: AR Peptidyl-prolyl cis-trans isomerases. Features: Kyphoscoliosis Myopathy Joint hypermobility Congenital hearing loss Atypical scarring and skin redundancy. Dordoni et al. 2016. Am. J. Med. Genet. 170 A: 2031 -8.

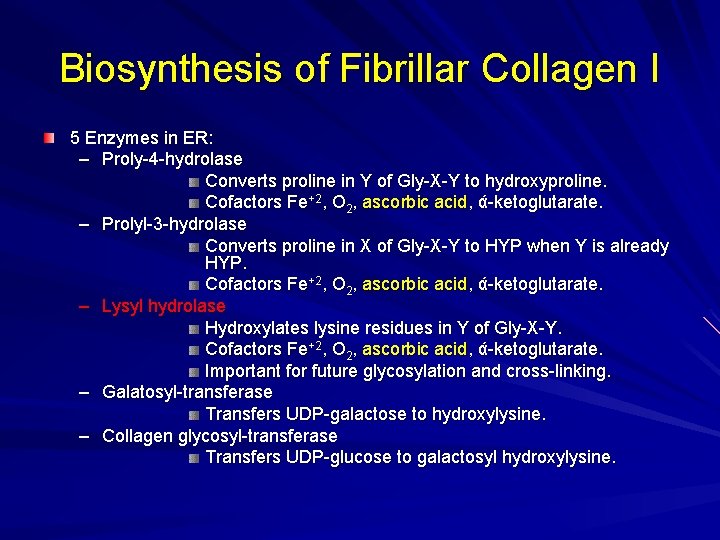
Biosynthesis of Fibrillar Collagen I 5 Enzymes in ER: – Proly-4 -hydrolase Converts proline in Y of Gly-X-Y to hydroxyproline. Cofactors Fe+2, O 2, ascorbic acid, ά-ketoglutarate. – Prolyl-3 -hydrolase Converts proline in X of Gly-X-Y to HYP when Y is already HYP. Cofactors Fe+2, O 2, ascorbic acid, ά-ketoglutarate. – Lysyl hydrolase Hydroxylates lysine residues in Y of Gly-X-Y. Cofactors Fe+2, O 2, ascorbic acid, ά-ketoglutarate. Important for future glycosylation and cross-linking. – Galatosyl-transferase Transfers UDP-galactose to hydroxylysine. – Collagen glycosyl-transferase Transfers UDP-glucose to galactosyl hydroxylysine.

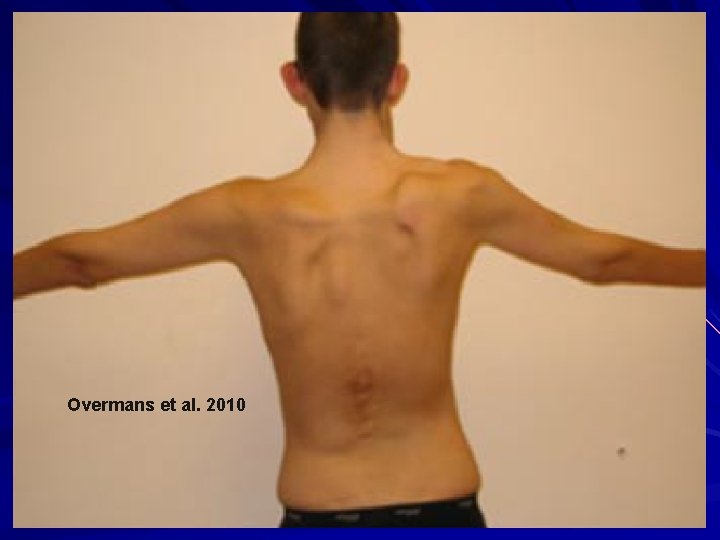
Overmans et al. 2010

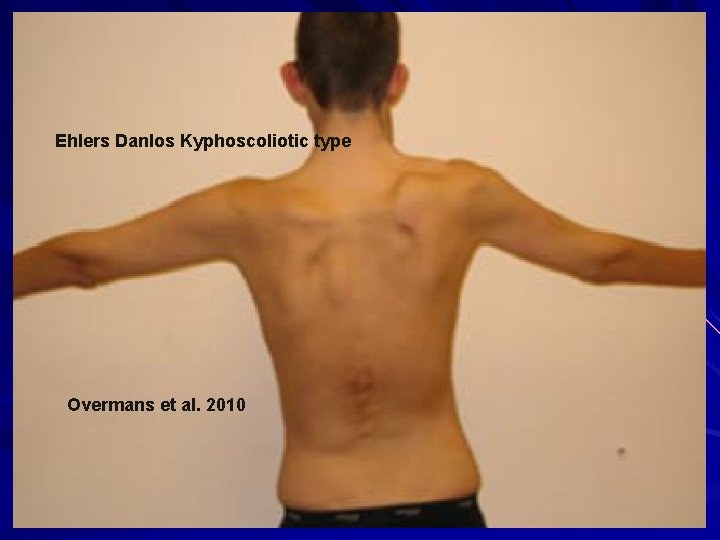
Ehlers Danlos Kyphoscoliotic type Overmans et al. 2010

EDS – Kyphoscoliotic (VIA) AR Deficiency of lysyl hydrolase type I (PLOD). Unable to tighten the final collagen molecule. hydroxylate lysines and hence can’t later cross-link them for collagen stability and insolubility. Clinical: Kyphoscoliosis at birth Scleral fragility with globe rupture/retinal detachment Severe muscular hypotonia at birth Generalized joint laxity +/- large artery rupture, atrophic scars, tissue fragility, marfanoid habitus, easy bruising, microcornea, osteopenia/osteoporosis Tosun et al. 2014. Pediatr. Neurol. 51: 566 -9; Overmans et al. 2010. Am. J. Med. Genet. 149 A: 2311 -16.

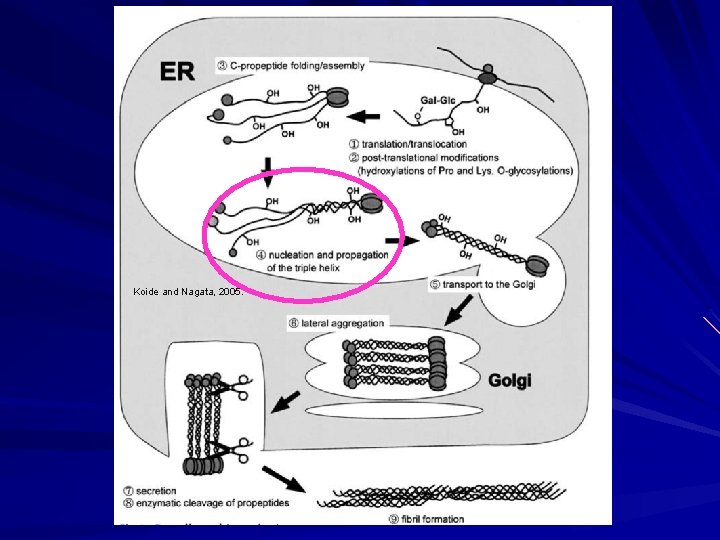
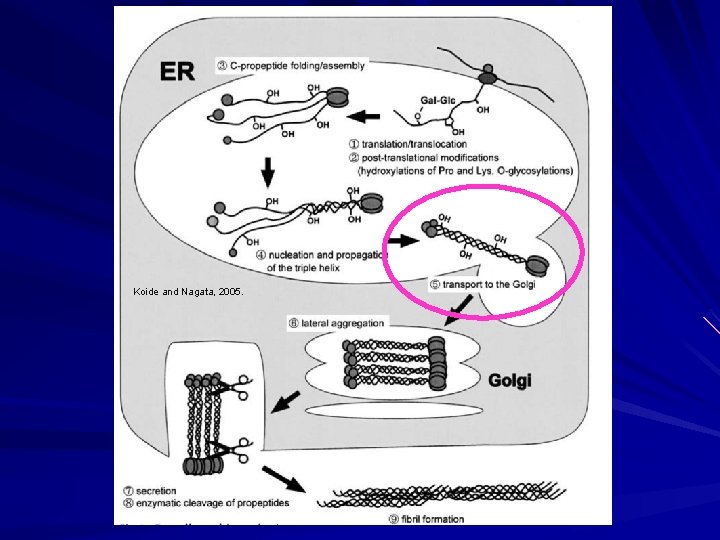
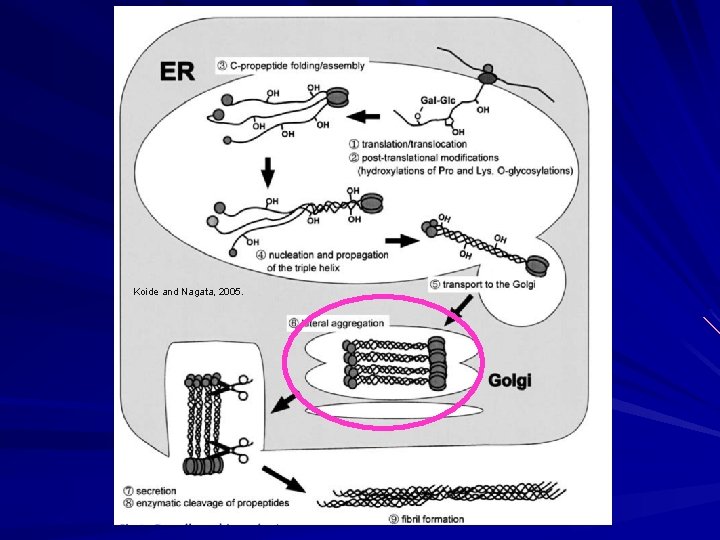
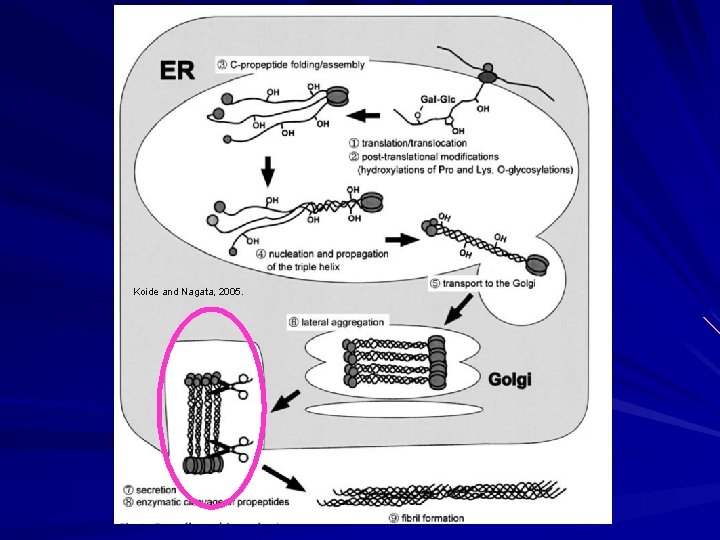
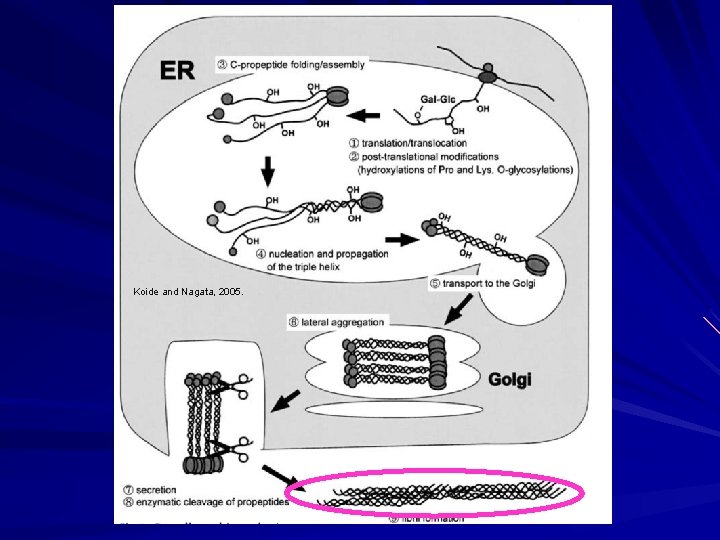
Biosynthesis of Fibrillar Collagen I Modified triple helix zippered up in ER. Secretion of Procollagen Aggregate laterally in golgi (loss of HSP 47) Secreted via golgi vacuoles along microtubules. Secreted into crypts around cell (fibripositors).

Koide and Nagata, 2005.

Koide and Nagata, 2005.

Koide and Nagata, 2005.

Koide and Nagata, 2005.

Koide and Nagata, 2005.

Koide and Nagata, 2005.

Koide and Nagata, 2005.

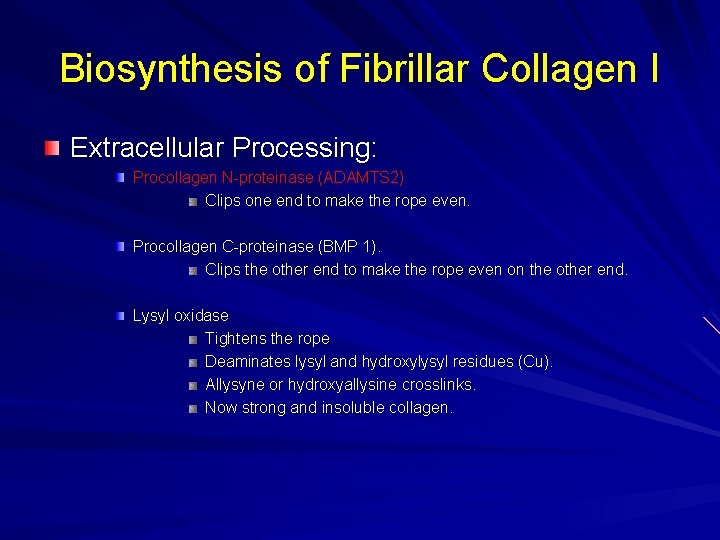
Biosynthesis of Fibrillar Collagen I Extracellular Processing: Procollagen N-proteinase (ADAMTS 2) Clips one end to make the rope even. Procollagen C-proteinase (BMP 1). Clips the other end to make the rope even on the other end. Lysyl oxidase Tightens the rope Deaminates lysyl and hydroxylysyl residues (Cu). Allysyne or hydroxyallysine crosslinks. Now strong and insoluble collagen.

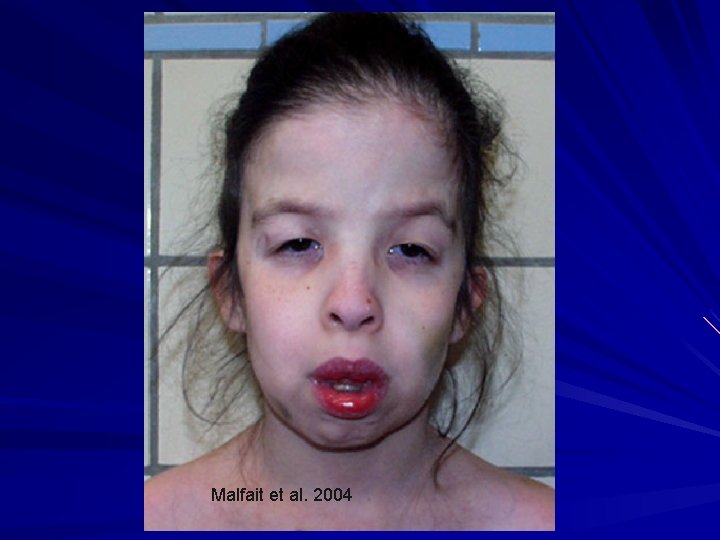
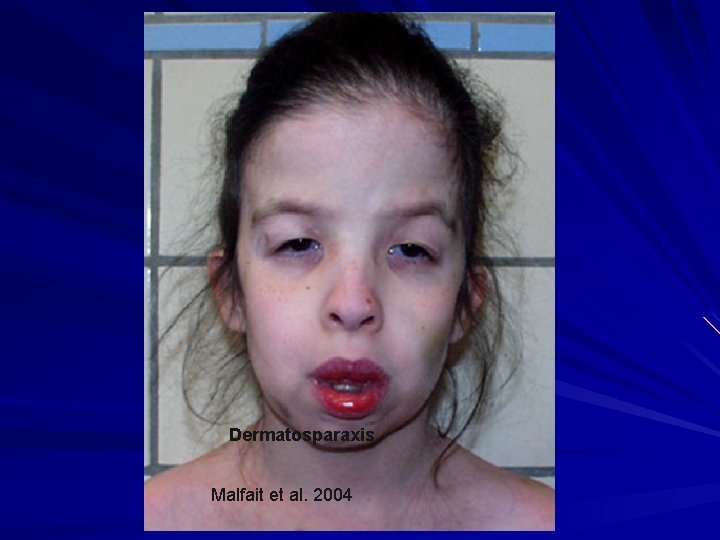
Malfait et al. 2004

Dermatosparaxis Malfait et al. 2004

EDS- Dermatosparaxis type (VIIC) AR – due to deficiency of procollagen I Nproteinase. With the rope frayed at one end, it breaks easily. Clinical: Severe skin fragility with avulsions Sagging, redundant skin (cutis laxa) +/- easy bruising, blue sclerae, PROM, joint laxity, umbilical hernias Van Damme et al. 2016. Genet. Med. 18: 882 -91; Malfait et al. 2004. Am. J. Med. Genet. 131 A: 18 -28.

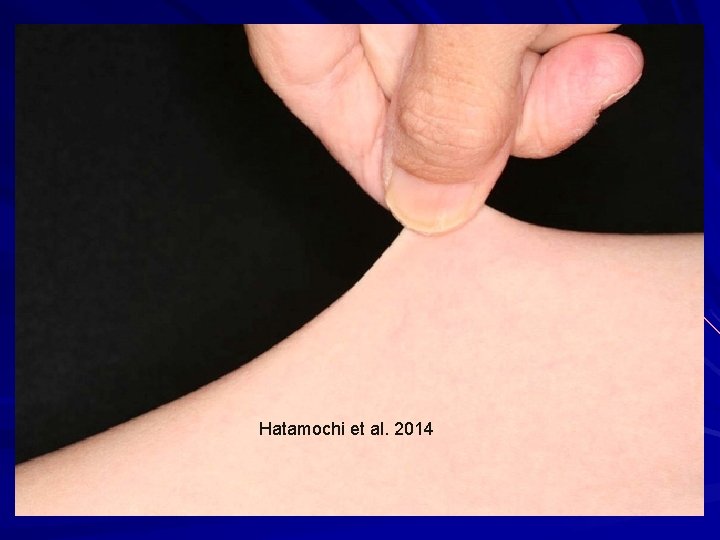
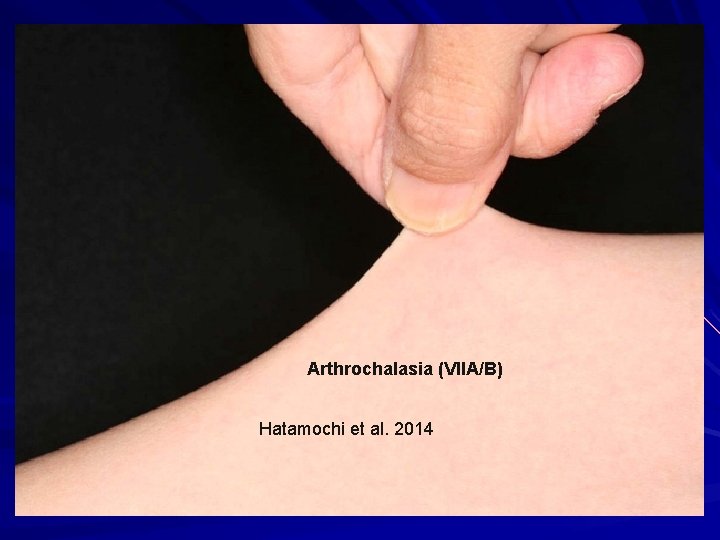
Hatamochi et al. 2014

Arthrochalasia (VIIA/B) Hatamochi et al. 2014

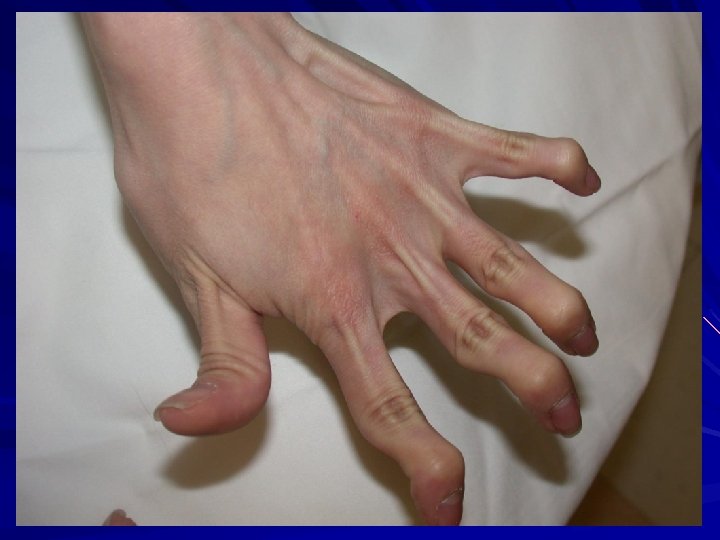
EDS-Arthrochalasia type (VIIA/B) AD – missing the marker of where to cut the rope, and so the end stays frayed. exon 6 (N-propeptide cleavage site) in either COL 1ά(I) or COL 1ά(II) genes. Clinical: Bilateral congenital hip dislocations Generalized and severe joint laxity +/- skin hyperextensibility, tissue fragility, atrophic scars, easy bruising, muscular hypotonia, wormian bones. N-propeptide remains associated and increases solubility of collagen and ↓feedback ↑collagen. Hatamochi et al. 2014. Gene 538: 199 -203; Klaassens et al. 2011. Clin. Genet. 82: 121 -30.

Collagen Biosynthesis Type V Collagen regulates fibrillogenesis. Type 5 at nucleus of fibre with binding sites pointing outwards, surrounded by types I and III with FACIT collagens XII and XIV to link to outside. Link to ground substance proteins, cell surface receptors and elastin.

The Dermis Collagen - gives strength Elastin - gives resilience Ground substance - gives texture.

Elastin: Continuous network from the epidermal junction to the hypodermis. Gives skin its resilience. Graduated amounts of mature elastin deposited on microfibrillar proteins. Effect on collagen regulation. Defects: Marfan's Syndrome, Cutis laxa, Menkes.

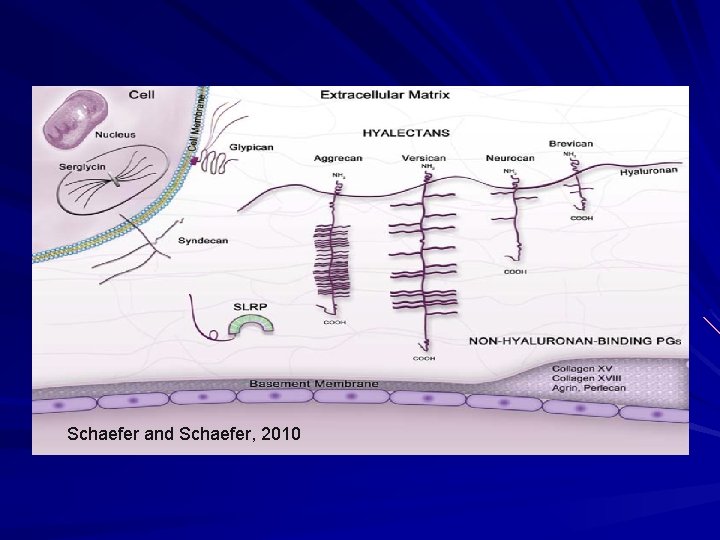
Ground Substance Amorphous material of the extra-cellular and extrafibrillar matrix. Binds water and maintains hydration of the dermis (texture). Composed of polysaccharides with negative charges. Glycosaminoglycans Proteoglycans (protein core with glycosaminoglycan) Proteins Many associate with collagens and can regulate the diameter of collagen fibrils.

Schaefer and Schaefer, 2010

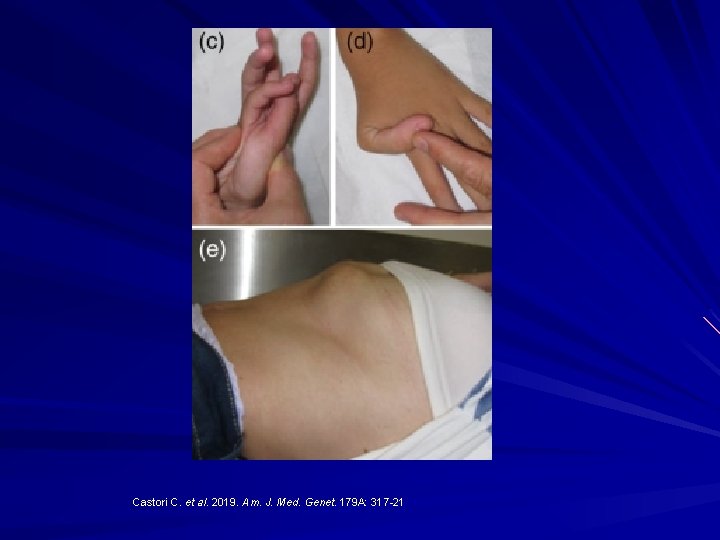
Castori C. et al. 2019. Am. J. Med. Genet. 179 A: 317 -21

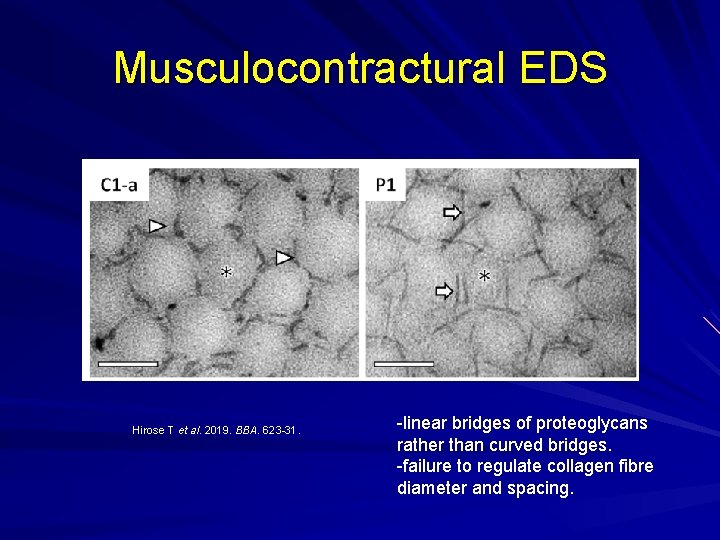
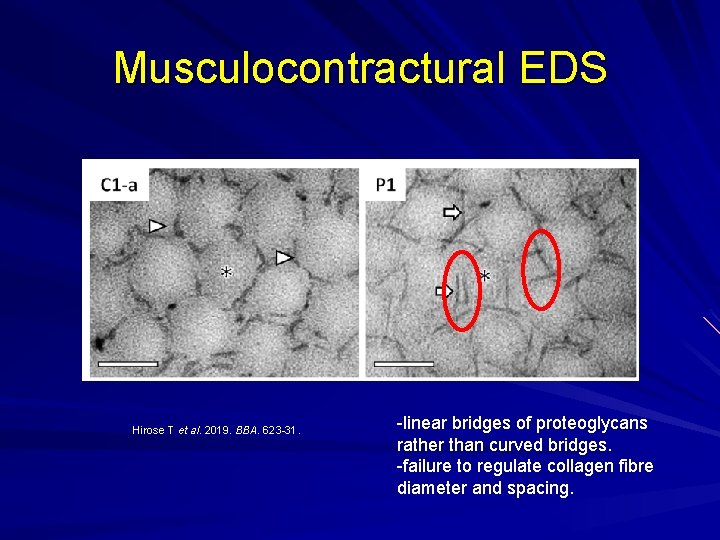
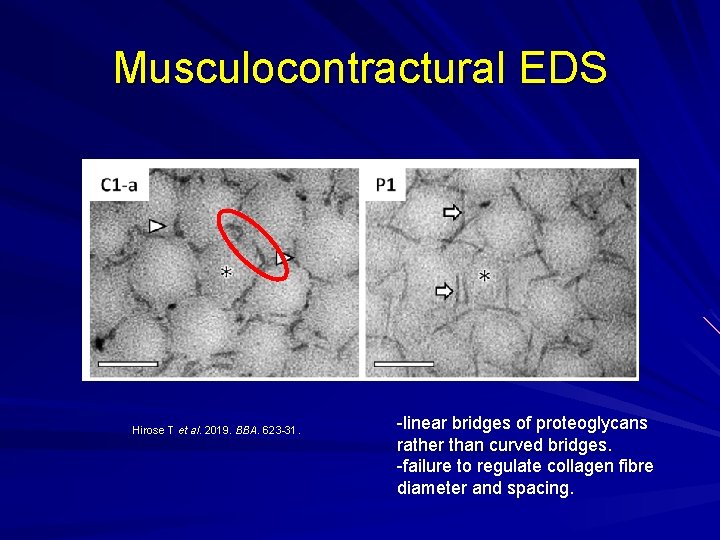
Musculocontractural EDS Enzyme carbohydrate sulfotransferase 14 (CHST 14) not working properly and one of the proteoglycans, decorin, doesn't get its proper glycanation and hence doesn't bind collagen properly. Myopathy with muscle contractures. Skin hyperextensibility Skin fragility Spinal deformities.

Musculocontractural EDS Hirose T et al. 2019. BBA. 623 -31. -linear bridges of proteoglycans rather than curved bridges. -failure to regulate collagen fibre diameter and spacing.

Musculocontractural EDS Hirose T et al. 2019. BBA. 623 -31. -linear bridges of proteoglycans rather than curved bridges. -failure to regulate collagen fibre diameter and spacing.

Musculocontractural EDS Hirose T et al. 2019. BBA. 623 -31. -linear bridges of proteoglycans rather than curved bridges. -failure to regulate collagen fibre diameter and spacing.

Syx D. et al. 2019. Human Mol. Gen. 28(11): 1853 -64.

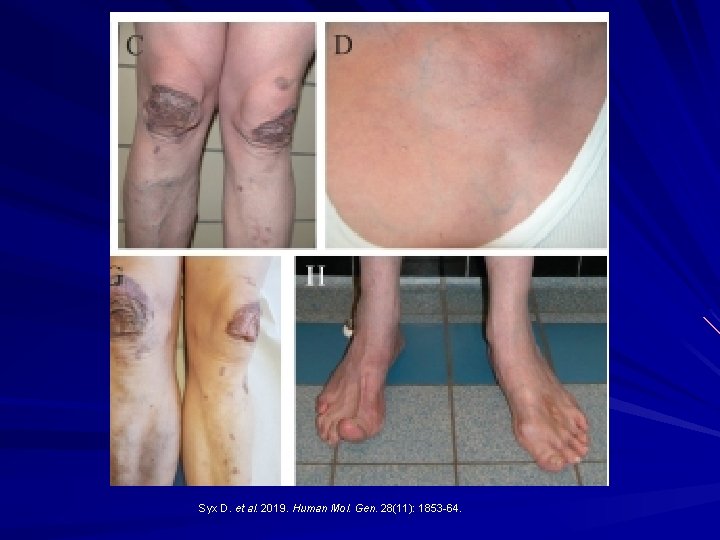
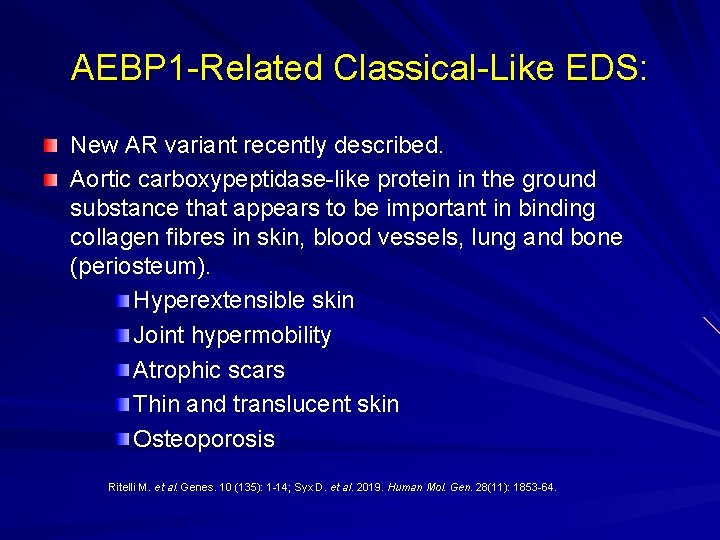
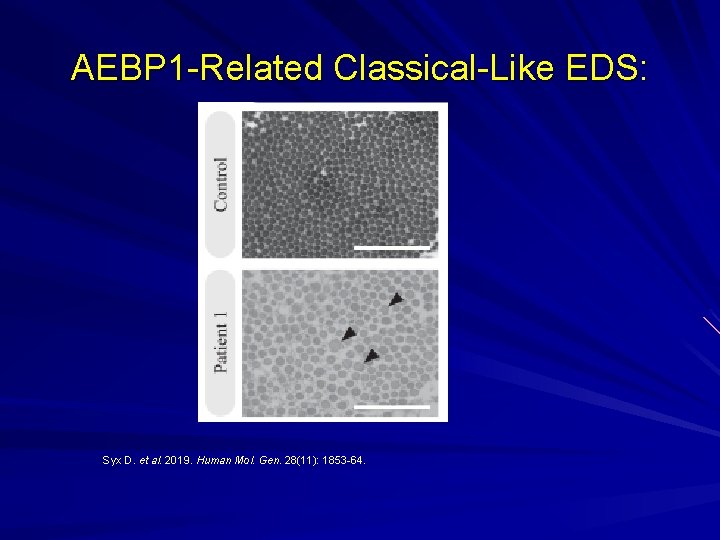
AEBP 1 -Related Classical-Like EDS: New AR variant recently described. Aortic carboxypeptidase-like protein in the ground substance that appears to be important in binding collagen fibres in skin, blood vessels, lung and bone (periosteum). Hyperextensible skin Joint hypermobility Atrophic scars Thin and translucent skin Osteoporosis Ritelli M. et al. Genes. 10 (135): 1 -14; Syx D. et al. 2019. Human Mol. Gen. 28(11): 1853 -64.

AEBP 1 -Related Classical-Like EDS: Syx D. et al. 2019. Human Mol. Gen. 28(11): 1853 -64.

AEBP 1 -Related Classical-Like EDS: Syx D. et al. 2019. Human Mol. Gen. 28(11): 1853 -64.

Ehlers Danlos Syndromes: Genes affected: – Fibrillar and non-fibrillar collagens. – Enzymes that process fibrillar collagens. – Glycosaminoglycan synthesis (interacts with collagen to regulate diameter). – Matrix proteins that influence collagen diameter.

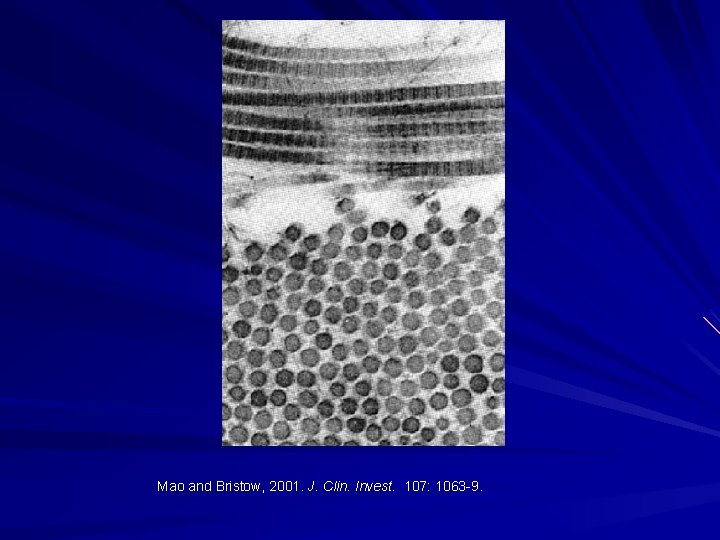
Role of Electron Microscopy: Normally collagen synthesis is tightly controlled with near identical size and spacing of molecules. Inherited disorders of connective tissue (IDCT) demonstrate variation in collagen size and interfibrillar spacing when assessed by TEM. Can be used to support the presence of an IDCT.

Ehlers Danlos Syndromes: Skin biopsy taken from upper inner arm or lower back/upper buttock (sun protected site). Glutaraldehyde and fixed for TEM. Magnification up to 500 000 X. Can assess variations in collagen fibrils.

Mao and Bristow, 2001. J. Clin. Invest. 107: 1063 -9.

Classic EDS Hausser and Anton-Lamprecht, 1994. Hum. Genet. 3: 394 -407.

Hausser and Anton-Lamprecht, 1994. Hum. Genet. 3: 394 -407.

Vascular type EDS Sobey, 2015. Arch. Dis. Child. 100: 57 -61.

Dermatosparaxis Van Damme et al. 2016. Genet. Med. 18: 882 -91; Malfait et al. 2004. Am. J. Med. Genet. 131 A: 18 -28.

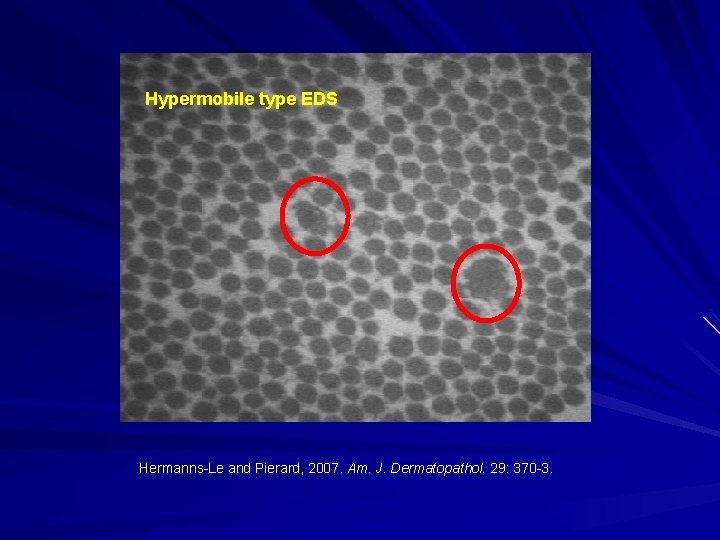
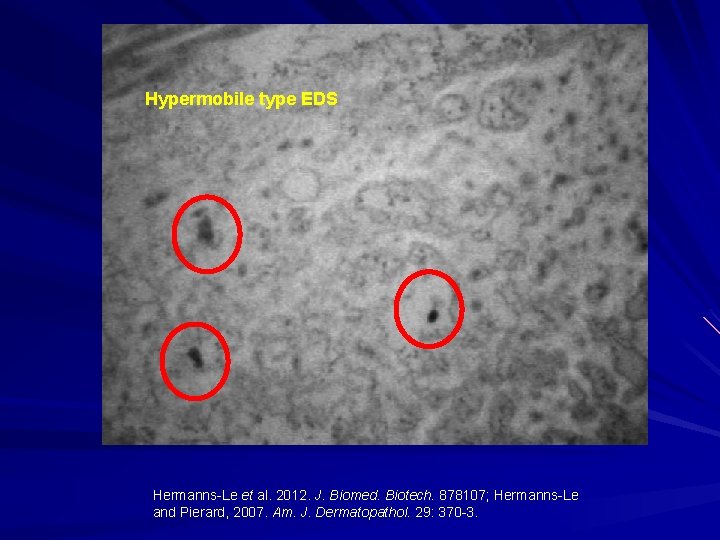
Hypermobile type EDS Hermanns-Le and Pierard, 2007. Am. J. Dermatopathol. 29: 370 -3.

Hypermobile type EDS Hermanns-Le et al. 2012. J. Biomed. Biotech. 878107; Hermanns-Le and Pierard, 2007. Am. J. Dermatopathol. 29: 370 -3.

Electron Microscopy and Collagen: EDS variants. Other inherited disorders of connective tissue: Osteogenesis imperfecta Caffey Disease Marfan's Syndrome Loeys-Dietz Pseudoxanthoma elasticum Cutis laxa Menkes Acquired disorders: Scleredema, scleromyxedema, nephrogenic fibrosing dermopathy.




Hypermobility Spectrum Disorder: Likely many disorders with common presentation. AD. Previously EDS Hypermobile type (subtype now only classified as EDS Hypermobile type). Features: Generalized joint hypermobility. Chronic joint and muscular pain. Recurrent joint dislocations or subluxations. Symptoms of autonomic dysfunction (dysautonomia). Easy bruising. No widened/papyraceous scars. Functional gastrointestinal disorders. Small fibre neuropathy Mast cell disorders (from flushing and itch to mast cell activation syndrome) Behaves like an inflammatory disorder in many patients. Martin A. 2019. Eur. J. Med. Genet. In press; Forghani I. 2019. Balkan Med. J. 36: 12 -6; Caston M. and Hakim A. 2017. Curr. Opin. Pediatr. 29(6): 640 -9.

Hypermobile type EDS: No single gene to date: Proteoglycans that regulate collagens. FACIT collagens. Elastin-related proteins.

Molecular Basis of HSD? Both HSD and h. EDS fibroblasts in contrast to both controls and other types of EDS show the exact same: Upregulation of specific wound receptors Upregulation or inflammatory mediators Disarray of extracellular matrix Prevalence of myofibroblast transition from fibroblasts (seen in chronic wound responses) not seen in other types of EDS. Zoppi N. et al. 2018. BBA. 1010 -1023.

Molecular Basis of HSD Other Roles of a "Normal Matrix": Migration and maturation of other matrical components (nerves, blood vessels, muscle, etc. ) Stabilization and activation of other cell types. Mast cells are intimate matrix cells in skin and gastrointestinal tract. Is there an abnormal effect from the matrix? Nerves are intimate with the matrix and could these be affected by the upregulated wound signals?

EDS Management from Dermatologic Perspective:

EDS - Management: Supplemental Vitamin C. Supplemental Vitamin A. Avoid glucocorticoids where possible. Protection from Trauma. Avoid contact sports. Avoid ASA. Vasopressin or transexaminic acid. Sobey, 2015. Arch. Dis. Child. 100: 57 -61; Morais et al. 2013. Acta Dermatovenerol. Croat. 21: 118 -22; Malfait et al. 2010. Genet. Med. 12: 597 -605; Mantle et al. 2005. Med. Hypoth. 64: 279 -83.

EDS - Management: Suturing wounds Double layer closures. Stitches held twice as long. Additional tape bandaging over wounds. Mast cell stabilization Cromolyn, ketotifen, monteleukast, omalizumab Antihistamines Sobey, 2015. Arch. Dis. Child. 100: 57 -61; Morais et al. 2013. Acta Dermatovenerol. Croat. 21: 118 -22; Mantle et al. 2005. Med. Hypoth. 64: 279 -83;

Summary: The skin matrix is a well-controlled interplay between: Collagen Elastin Ground Substance Electron microscopy can detect abnormalities in matrical structure and support a diagnosis of an inherited disorders of connective tissue. Hypermobility Spectrum Disorder has changes in the matrix that likely can exert effect on both the migration and subsequent activity of other cell types.