GCSE Chemistry and DAS Chemistry Support Events Support
































































- Slides: 119

GCSE Chemistry and DAS Chemistry Support Events Support for teaching the practical aspects of the specification January 2019

Welcome & Introductions • Elaine Lennox – Subject Officer for Chemistry and Double Award Science • Alyn Mc. Farland – Chief Examiner for GCSE Chemistry • Bob Cummings – Chief Examiner for GCSE DAS Chemistry

Agenda Support Events of GCSE Chemistry and DAS Chemistry (for first teaching September 2017) Elaine Lennox – General Information ü Curriculum Monitoring ü CCEA Analytics ü New Grading System ü General specification information ü Commonalities between the GCSE Chemistry and DAS Chemistry specifications ü Resources available

Agenda • Reflection of the Summer 2018 series – Unit 1 Grade Boundaries – Alyn Mc. Farland: GCSE Chemistry – Bob Cummings: GCSE DAS Chemistry • The Practical Skills Unit – General Information and Skills Assessed – Alyn Mc. Farland: GCSE Chemistry – Bob Cummings: GCSE DAS Chemistry • Q & A


• Online questionnaires (a series of 5 min questionnaires for all teachers) • School visits to hear from a wide range of teachers and leaders within schools • ALC Principals’ meetings • Subject specific panels of key stakeholders • Pupil interviews • Parent consultation through partnership with parenting organisations • Different schools will be involved in different aspects. Randomly selected schools and ALCs will be contacted by email.

• To start professional discussions • A systematic programme of listening to empower teacher voice • To inform planning for support • To identify gaps and connections in the curriculum • Inform CCEA’s programme of support for 2019/20

For further information, please contact: Post-primary Róisín Mc. Creesh T: 02890261200 ext: 2635 M: 07970204356 E: rmccreesh@ccea. org. uk Primary Emma Holmes T: 02890261200 ext: 2670 M: 07799346870 E: eholmes@ccea. org. uk

CCEA Analytics Ø Online application providing comparative data to benchmark performance Ø Summer 2018 data available from November 2018 Ø Access to CCEA Analytics is at Head of Centre’s discretion Ø Exams Officer registers users via the Central Login Ø Visit the CCEA website (Qualifications>Analytics) to: Ø get more information (including contacts) Ø access the application Ø provide feedback

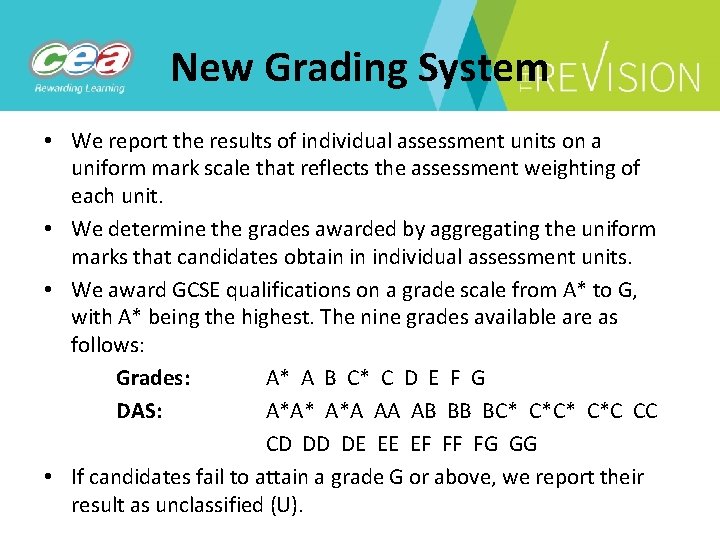
New Grading System • We report the results of individual assessment units on a uniform mark scale that reflects the assessment weighting of each unit. • We determine the grades awarded by aggregating the uniform marks that candidates obtain in individual assessment units. • We award GCSE qualifications on a grade scale from A* to G, with A* being the highest. The nine grades available are as follows: Grades: A* A B C* C D E F G DAS: A*A* A*A AA AB BB BC* C*C CC CD DD DE EE EF FF FG GG • If candidates fail to attain a grade G or above, we report their result as unclassified (U).

GCSE Unitised Qualifications • All unitised GCSE qualifications use uniform marks (ums) • This ensures fairness year on year irrespective of the demand of the paper/ms • The total number of uniform marks for a unitised GCSE is 400 um and 600 um for DAS

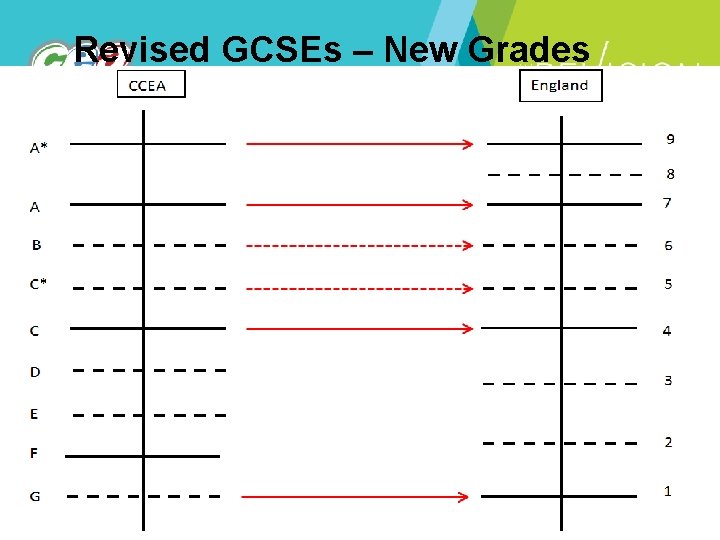
Revised GCSEs – New Grades

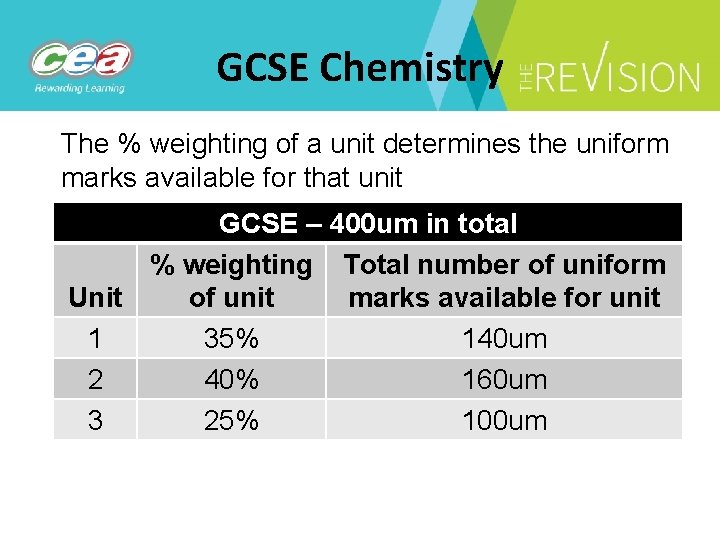
GCSE Chemistry The % weighting of a unit determines the uniform marks available for that unit GCSE – 400 um in total % weighting Total number of uniform Unit of unit marks available for unit 1 35% 140 um 2 40% 160 um 3 25% 100 um

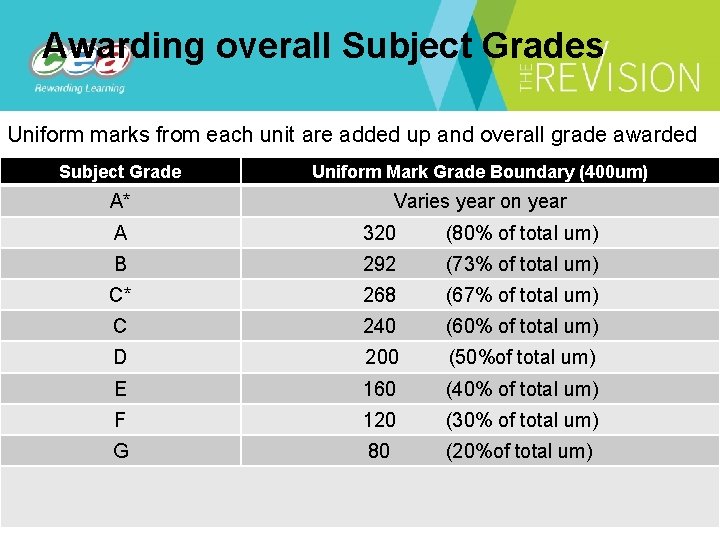
Awarding overall Subject Grades Uniform marks from each unit are added up and overall grade awarded Subject Grade Uniform Mark Grade Boundary (400 um) A* Varies year on year A 320 (80% of total um) B 292 (73% of total um) C* 268 (67% of total um) C 240 (60% of total um) D 200 (50%of total um) E 160 (40% of total um) F 120 (30% of total um) G 80 (20%of total um)

Unit 1 Total uniform marks for unit = 140 (35% weighting at GCSE) Higher Tier grade HT um grade Foundation Tier grade boundary FT um grade boundary A 112 B 102 C* 94 C 84 D 70 Allowable E (45%) of available um 63 E 56 U Less than 63 F 42 G 28 Highest um achievable in foundation = 101

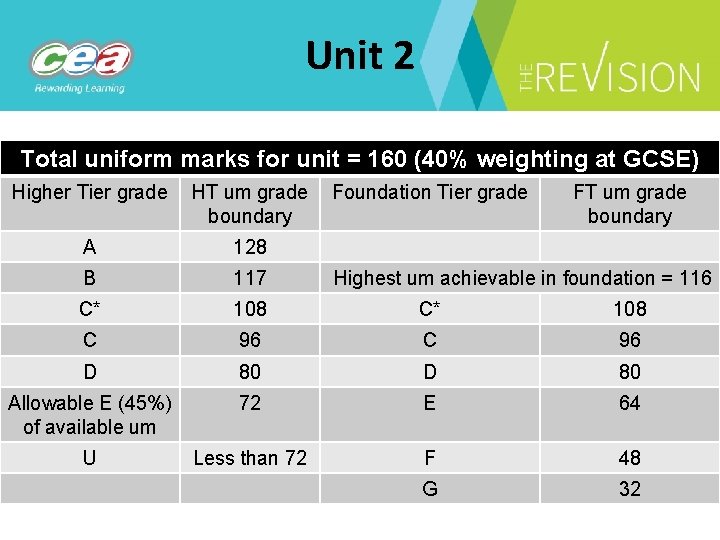
Unit 2 Total uniform marks for unit = 160 (40% weighting at GCSE) Higher Tier grade HT um grade Foundation Tier grade boundary FT um grade boundary A 128 B 117 C* 108 C 96 D 80 Allowable E (45%) of available um 72 E 64 U Less than 72 F 48 G 32 Highest um achievable in foundation = 116

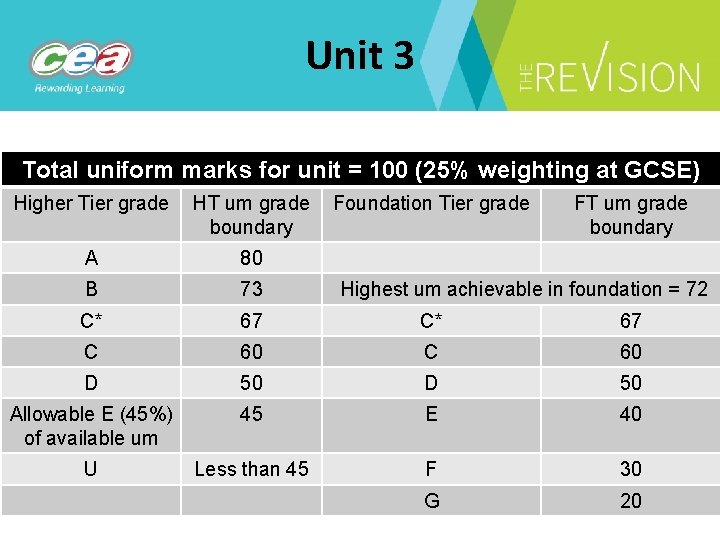
Unit 3 Total uniform marks for unit = 100 (25% weighting at GCSE) Higher Tier grade HT um grade Foundation Tier grade boundary FT um grade boundary A 80 B 73 C* 67 C 60 D 50 Allowable E (45%) of available um 45 E 40 U Less than 45 F 30 G 20 Highest um achievable in foundation = 72

GCSE DAS: Chemistry The % weighting of a unit determines the uniform marks available for that unit GCSE – 600 um in total % weighting Total number of uniform Unit of unit marks available for unit 1 11% x 3 66 um 2 14% x 3 84 um 7 25% 150 um

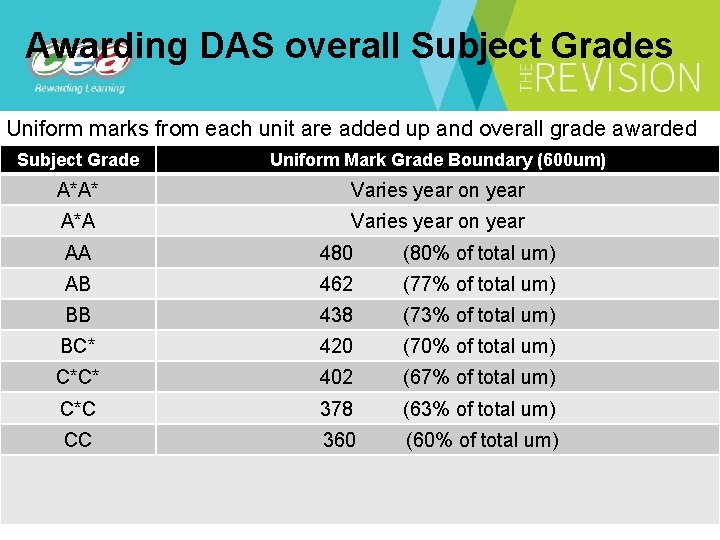
Awarding DAS overall Subject Grades Uniform marks from each unit are added up and overall grade awarded Subject Grade Uniform Mark Grade Boundary (600 um) A*A* Varies year on year A*A Varies year on year AA 480 (80% of total um) AB 462 (77% of total um) BB 438 (73% of total um) BC* 420 (70% of total um) C*C* 402 (67% of total um) C*C 378 (63% of total um) CC 360 (60% of total um)

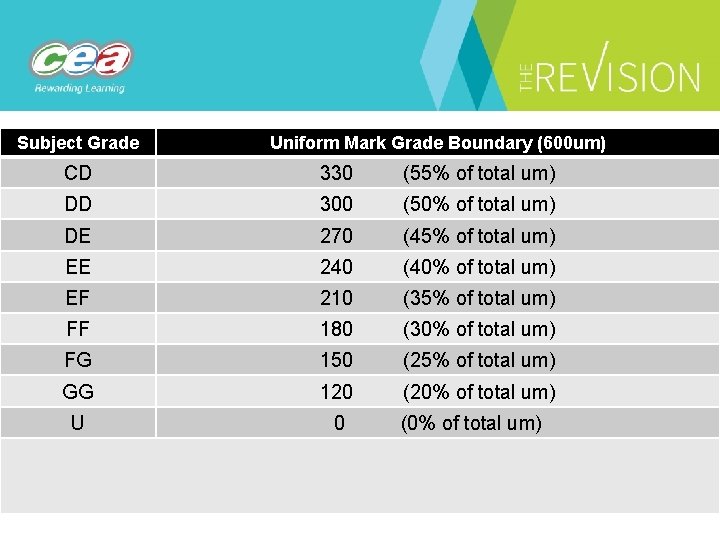
Subject Grade Uniform Mark Grade Boundary (600 um) CD 330 (55% of total um) DD 300 (50% of total um) DE 270 (45% of total um) EE 240 (40% of total um) EF 210 (35% of total um) FF 180 (30% of total um) FG 150 (25% of total um) GG 120 (20% of total um) U 0 (0% of total um)

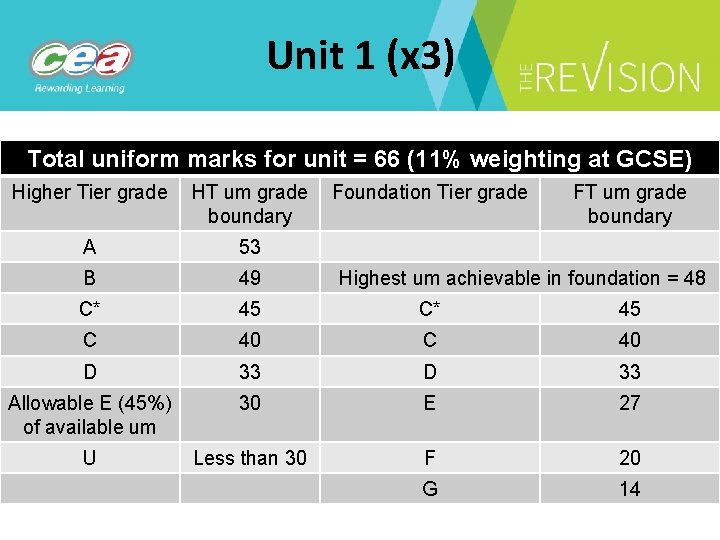
Unit 1 (x 3) Total uniform marks for unit = 66 (11% weighting at GCSE) Higher Tier grade HT um grade Foundation Tier grade boundary FT um grade boundary A 53 B 49 C* 45 C 40 D 33 Allowable E (45%) of available um 30 E 27 U Less than 30 F 20 G 14 Highest um achievable in foundation = 48

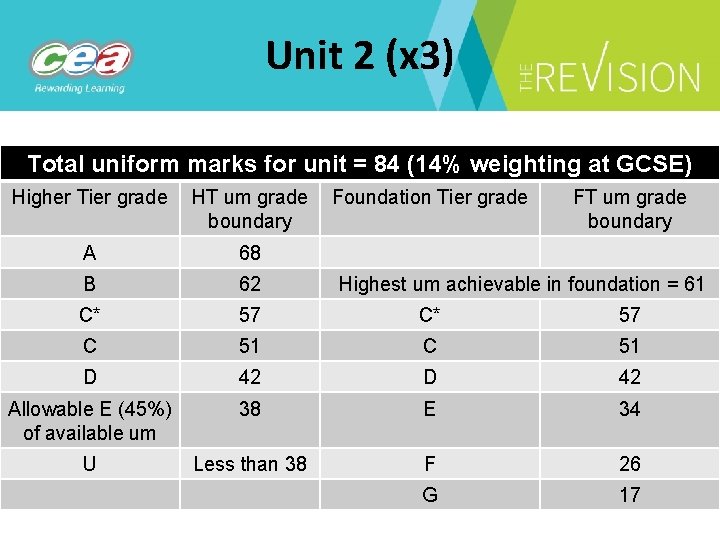
Unit 2 (x 3) Total uniform marks for unit = 84 (14% weighting at GCSE) Higher Tier grade HT um grade Foundation Tier grade boundary FT um grade boundary A 68 B 62 C* 57 C 51 D 42 Allowable E (45%) of available um 38 E 34 U Less than 38 F 26 G 17 Highest um achievable in foundation = 61

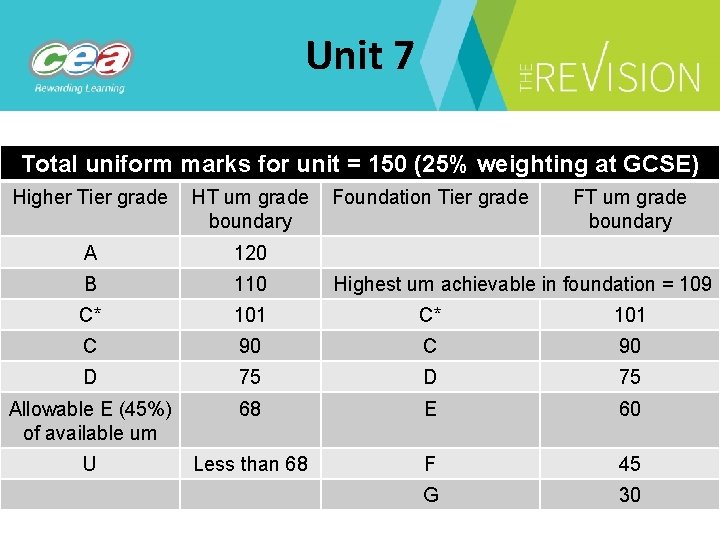
Unit 7 Total uniform marks for unit = 150 (25% weighting at GCSE) Higher Tier grade HT um grade Foundation Tier grade boundary FT um grade boundary A 120 B 110 C* 101 C 90 D 75 Allowable E (45%) of available um 68 E 60 U Less than 68 F 45 G 30 Highest um achievable in foundation = 109

Realignment of Outcomes • No A* at unit level – only calculated at qualification level. Likely to see a dip in these. • Fewer Grade B’s • New C* - bottom end of Grade B and top end of Grade C • Fewer Grade C’s • Practical Unit Outcomes to be reduced as its more demanding, this will be compensated in higher outcomes in theory units.

General Specification Information: GCSE Chemistry Content Assessment • Divided into 3 units: • Unit 1 – Structures, Trends, Chemical Reactions, Quantitative Chemistry and Analysis (35%) • Unit 2: Further Chemical Reactions, Rates and Equilibrium, Calculations and Organic Chemistry (40%) • External written examination • Foundation and Higher Tiers • Foundation Tier: 1 h (60 marks) • Higher Tier 1 h 15 m (80 marks) • • External written examination Foundation and Higher Tiers Foundation Tier 1 h 15 m (80 marks) Higher Tiers 1 h 30 m (100 marks)

Content Assessment • Unit 3: Practical Skills (25%) • Booklet A practical assessment (7. 5%) • Foundation and Higher Tiers • 2 hours (30 marks) • Booklet B practical theory examination (17. 5%) • Foundation and Higher Tiers • 1 hour (70 marks) • Unit grades: • Higher Tier: A* - D/E • Foundation Tier: C* - G

General Specification Information: DAS Chemistry Content Assessment • Divided into 7 units: • Unit 1 – (11% each) B 1: Cells, Living Processes and Biodiversity C 1: Structures, Trends, Chemical Reactions, Quantitative Chemistry and Analysis P 1: Motion, Force, Moments, Energy, Density, Kinetic Theory, Radioactivity, Nuclear Fission and Fusion • External written examination • Foundation and Higher Tiers • Foundation Tier: 1 h (60 marks) • Higher Tier 1 h (70 marks)

Content Assessment • Unit 2 - (14% each) • • B 2 – Body Systems, Genetics, Microorganisms and Health C 2 – Further Chemical Reactions, Rates and Equilibrium, Calculations and Organic Chemistry P 2 – Waves, Light, Electricity, Magnetism, Electromagnetism and Space Physics External written examination Foundation and Higher Tiers Foundation Tier 1 h 15 m (70 marks) Higher Tier 1 h 15 m (80 marks)

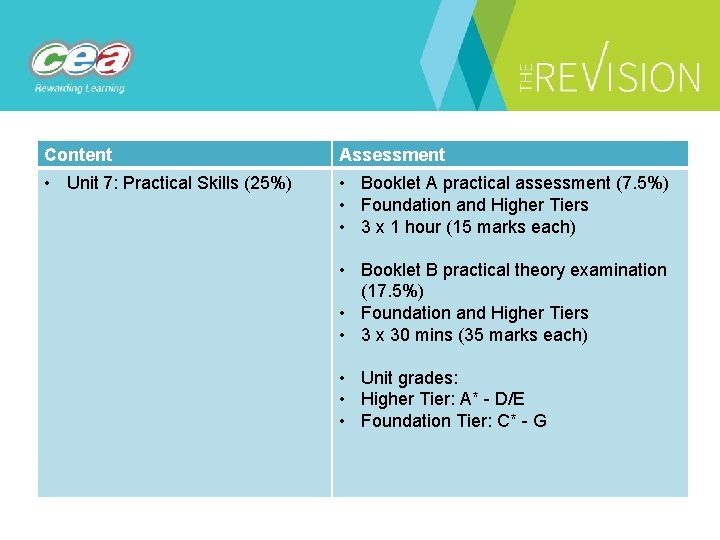
Content Assessment • Unit 7: Practical Skills (25%) • Booklet A practical assessment (7. 5%) • Foundation and Higher Tiers • 3 x 1 hour (15 marks each) • Booklet B practical theory examination (17. 5%) • Foundation and Higher Tiers • 3 x 30 mins (35 marks each) • Unit grades: • Higher Tier: A* - D/E • Foundation Tier: C* - G

Commonalities • GCSE DAS Chemistry is a subset of the GCSE Chemistry specification • The 6 prescribed practicals on the DAS specification are taken directly from the 9 prescribed practicals on the GCSE Chemistry specification • Question styles are similar • Practical Booklet A and B assess similar practical skills

Support • Direct access to Subject Officer • Dedicated microsite with News and Events • Specification • Specimen Assessment materials • Past papers and Mark Schemes • Factfiles for every topic • Answers to Factfile Questions (coming soon) • Practical Manuals • A document listing all practical opportunities to support Booklet B • Glossary of terms • Command Words Guide (DAS only coming soon) • Practical Skills Glossary (DAS only coming soon) • Exemplifying Examination Performance materials • Textbooks and Revision Guides (not CCEA endorsed) Teacher Support • Editable Planning Framework (scheme of work) • Student Guide • Grade Boundaries and Outcomes • CCEA dedicated BBC Bitesize (coming soon) • Centre Support visits • Support Days

During discussions, please avoid mentioning specifics of live practical materials. REFLECTION OF THE SUMMER 2018 SERIES

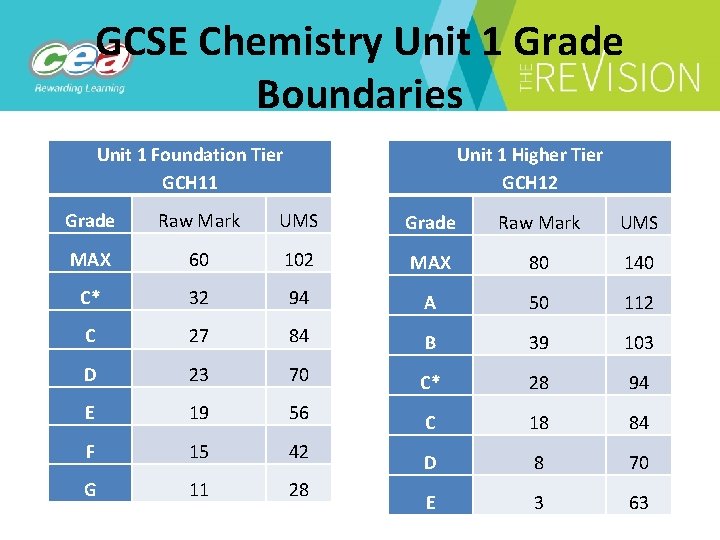
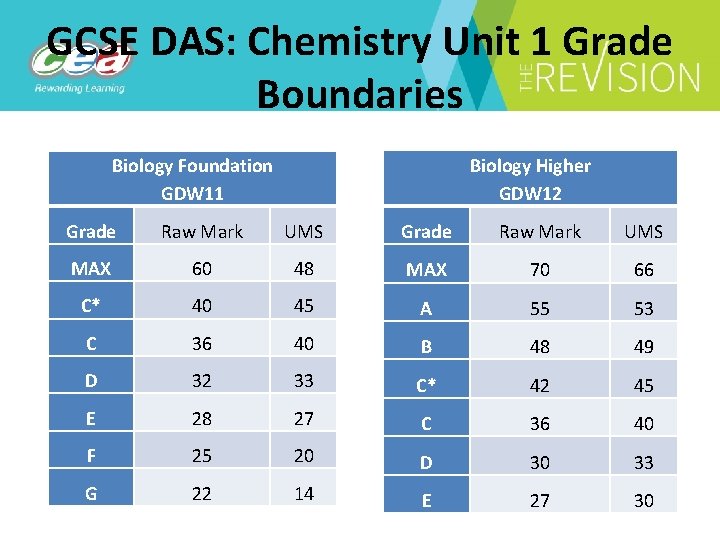
GCSE Chemistry Unit 1 Grade Boundaries Unit 1 Foundation Tier GCH 11 Unit 1 Higher Tier GCH 12 Grade Raw Mark UMS MAX 60 102 MAX 80 140 C* 32 94 A 50 112 C 27 84 B 39 103 D 23 70 C* 28 94 E 19 56 C 18 84 F 15 42 D 8 70 G 11 28 E 3 63

GCSE DAS: Chemistry Unit 1 Grade Boundaries Biology Higher GDW 12 Biology Foundation GDW 11 Grade Raw Mark UMS MAX 60 48 MAX 70 66 C* 40 45 A 55 53 C 36 40 B 48 49 D 32 33 C* 42 45 E 28 27 C 36 40 F 25 20 D 30 33 G 22 14 E 27 30

Chemistry Foundation GDW 21 Chemistry Higher GDW 22 Grade Raw Mark UMS MAX 60 48 MAX 70 66 C* 42 45 A 48 53 C 34 40 B 41 49 D 28 33 C* 34 45 E 22 27 C 28 40 F 16 20 D 22 33 G 10 14 E 19 30

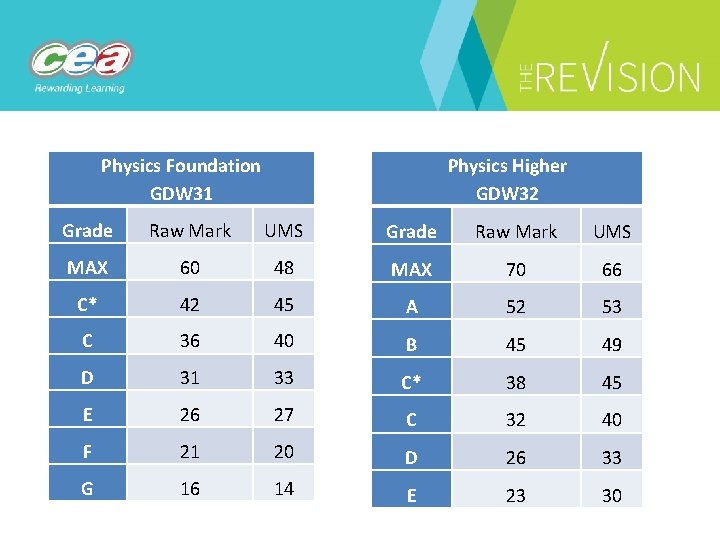
Physics Higher GDW 32 Physics Foundation GDW 31 Grade Raw Mark UMS MAX 60 48 MAX 70 66 C* 42 45 A 52 53 C 36 40 B 45 49 D 31 33 C* 38 45 E 26 27 C 32 40 F 21 20 D 26 33 G 16 14 E 23 30

Alyn Mc. Farland GCSE CHEMISTRY

Key Areas • • • Item Level Data Mean per question across all candidates New content Mathematics QWC

Lowest mean questions – all <40% Mean = 0. 14 (14%) Mean = 0. 8 (27%) Mean = 0. 27 (27%)

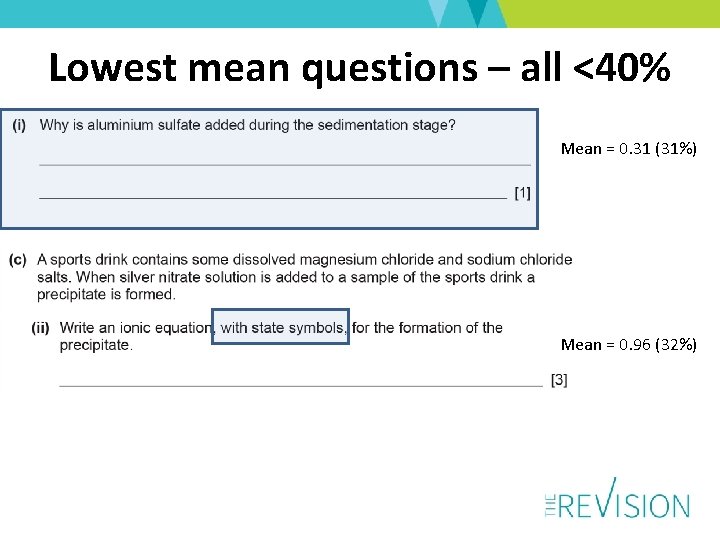
Lowest mean questions – all <40% Mean = 0. 31 (31%) Mean = 0. 96 (32%)

Lowest mean questions – all <40% Mean = 0. 77 (39%)

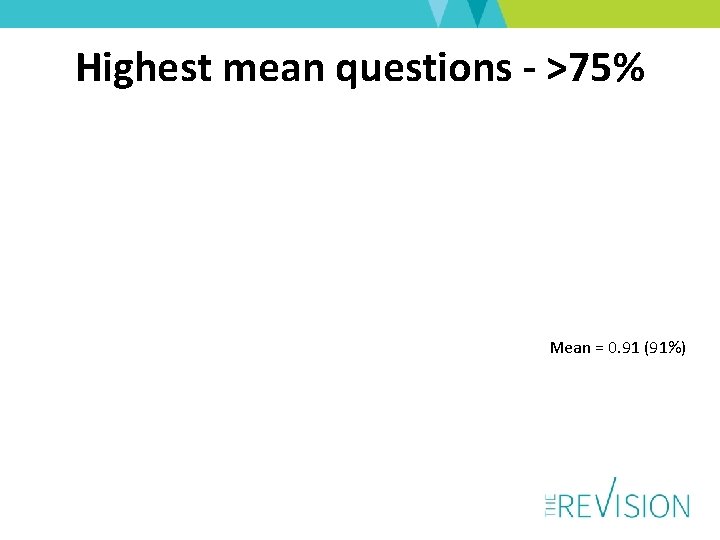
Highest mean questions - >75% Mean = 0. 91 (91%)

Highest mean questions - >75% Mean = 1. 76 (88%) Mean = 0. 87 (87%)

Highest mean questions - >75% Mean = 0. 77 (77%) Mean = 0. 75 (75%)

New content questions Mean = 1. 51 (50%) Mean = 0. 69 (69%)

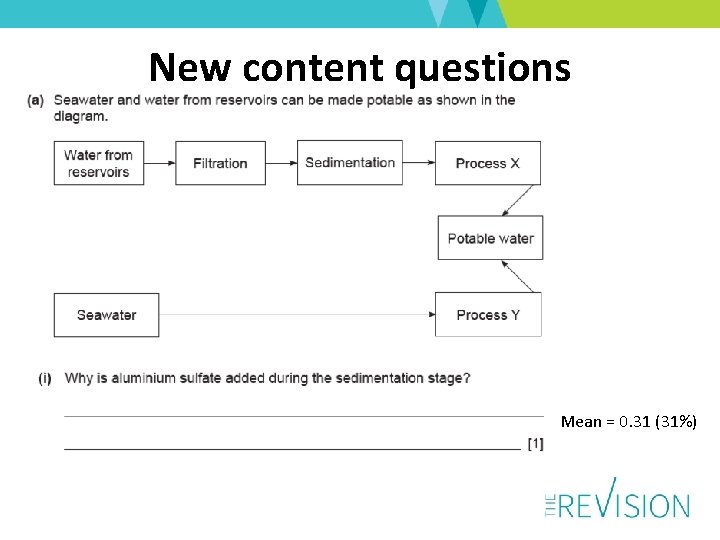
New content questions Mean = 0. 31 (31%)

New content questions Mean = 1. 01 (51%) Mean = 0. 43 (43%) Mean = 0. 14 (14%)

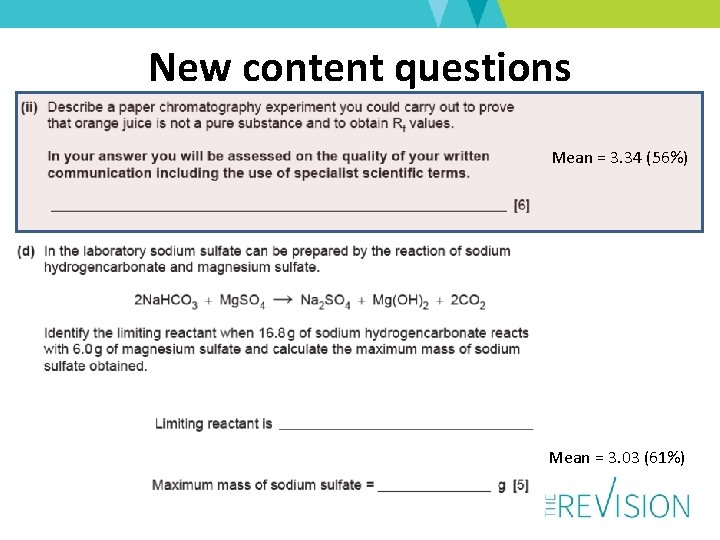
New content questions Mean = 3. 34 (56%) Mean = 3. 03 (61%)

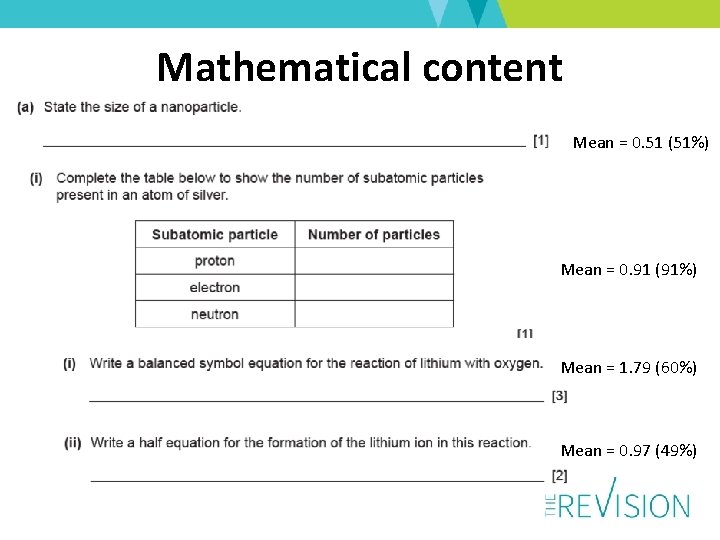
Mathematical content Mean = 0. 51 (51%) Mean = 0. 91 (91%) Mean = 1. 79 (60%) Mean = 0. 97 (49%)

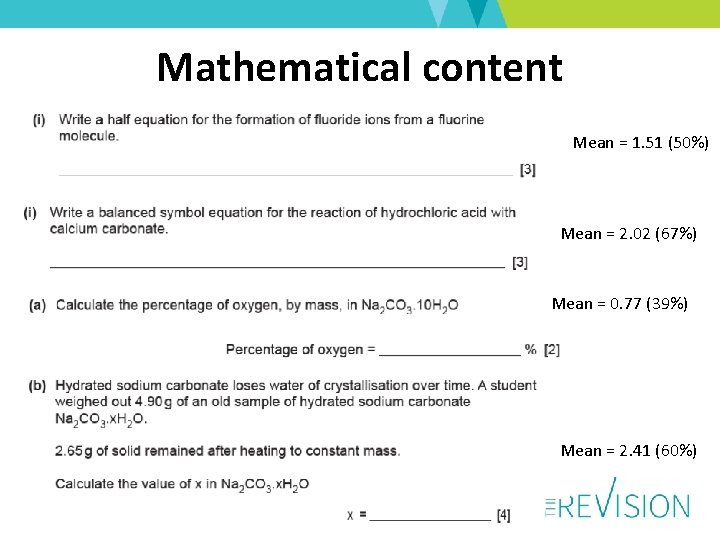
Mathematical content Mean = 1. 51 (50%) Mean = 2. 02 (67%) Mean = 0. 77 (39%) Mean = 2. 41 (60%)

Mathematical content Mean = 1. 59 (53%) Mean = 3. 03 (61%)

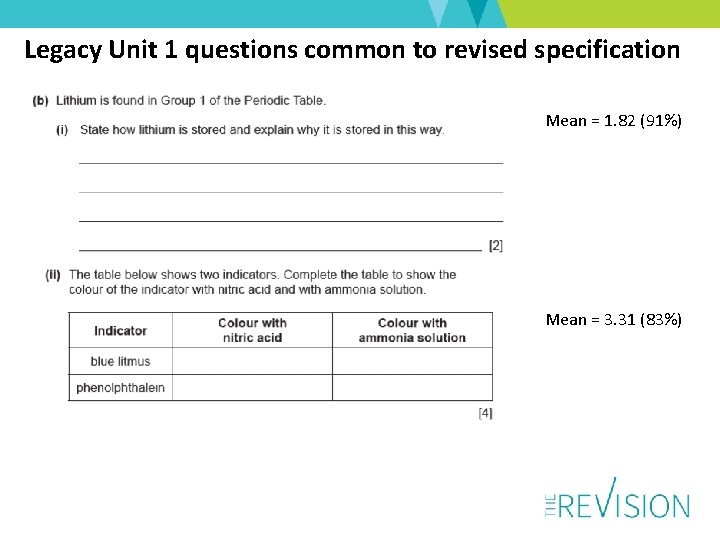
Legacy Unit 1 questions common to revised specification Mean = 1. 82 (91%) Mean = 3. 31 (83%)

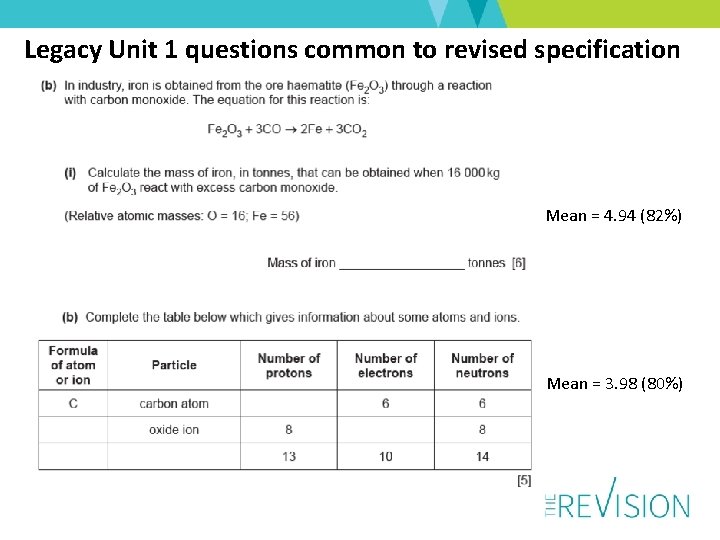
Legacy Unit 1 questions common to revised specification Mean = 4. 94 (82%) Mean = 3. 98 (80%)

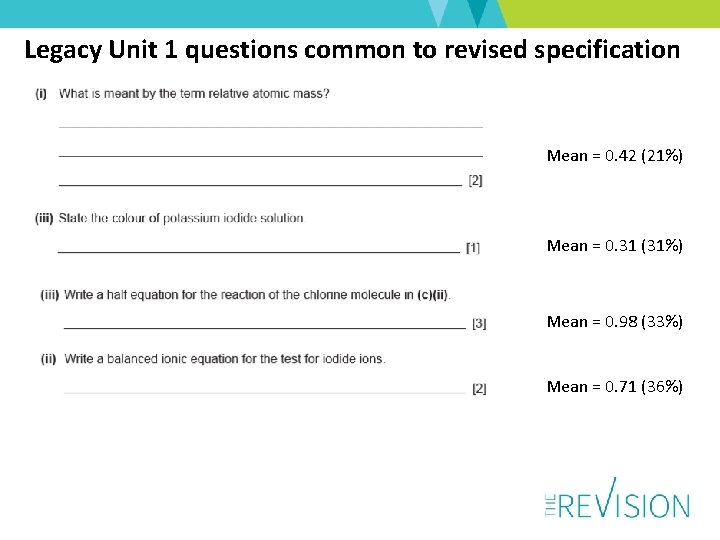
Legacy Unit 1 questions common to revised specification Mean = 0. 42 (21%) Mean = 0. 31 (31%) Mean = 0. 98 (33%) Mean = 0. 71 (36%)

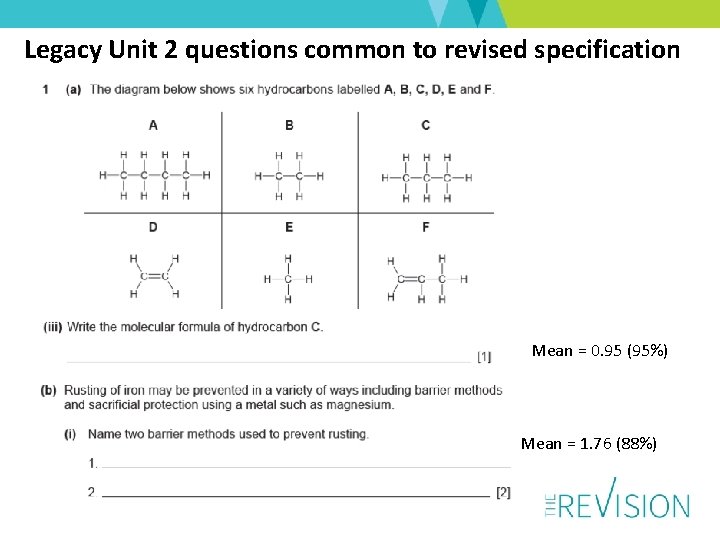
Legacy Unit 2 questions common to revised specification Mean = 0. 95 (95%) Mean = 1. 76 (88%)

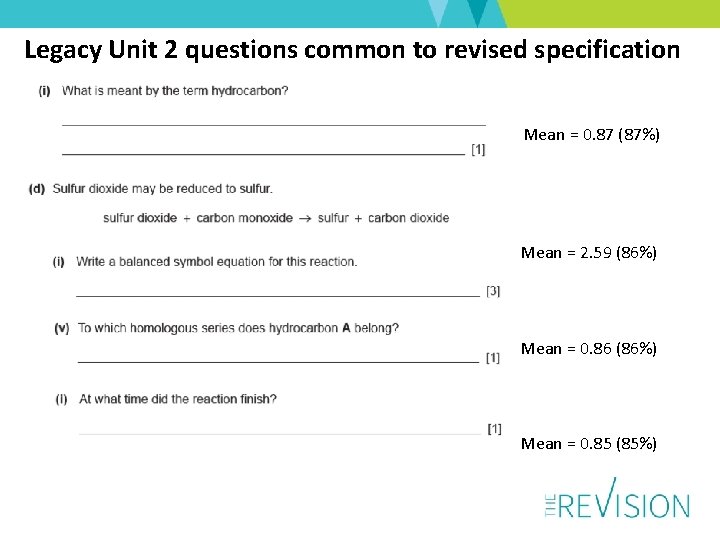
Legacy Unit 2 questions common to revised specification Mean = 0. 87 (87%) Mean = 2. 59 (86%) Mean = 0. 86 (86%) Mean = 0. 85 (85%)

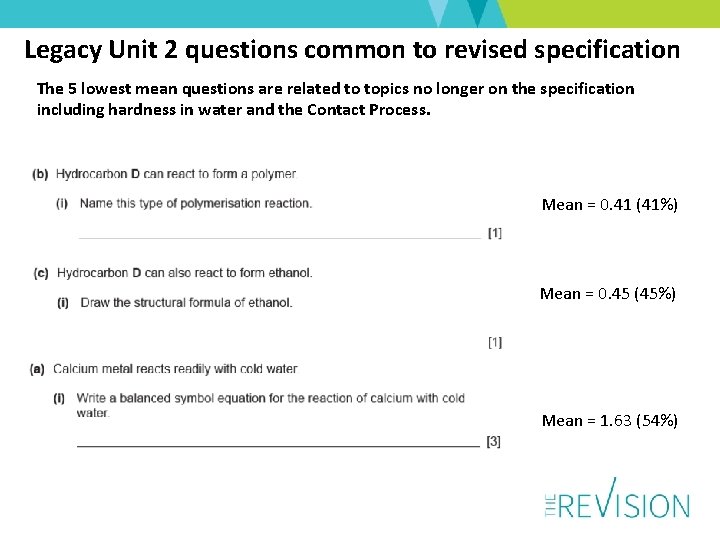
Legacy Unit 2 questions common to revised specification The 5 lowest mean questions are related to topics no longer on the specification including hardness in water and the Contact Process. Mean = 0. 41 (41%) Mean = 0. 45 (45%) Mean = 1. 63 (54%)

Bob Cummings GCSE DAS: CHEMISTRY

Analysis of the May 2018 GDW 22 and 21 papers • An overview of GDW 22 – where did candidates score well or poorly? • Question by question – what insights does this analysis provide? • Was new content a factor? • Was the increased Mathematical demand a factor? • An overview of GDW 21 – where did candidates score well or poorly? • Any insights regarding choosing Tiers of Entry?

GDW 22 (Higher Tier) May 2018 – Analysis

Q 1 – New Material – not well answered - 43% got multiple choice part correct !! (b)(I) 25% scored 0; b(ii) 50% knew the flame colour

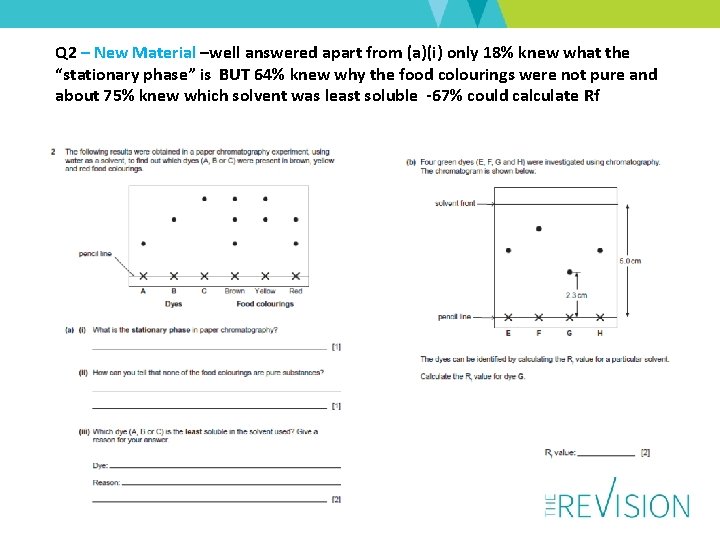
Q 2 – New Material –well answered apart from (a)(i) only 18% knew what the “stationary phase” is BUT 64% knew why the food colourings were not pure and about 75% knew which solvent was least soluble -67% could calculate Rf

Q 3 – New Material –well answered apart from (a)- only 43% could recall the size of a nanoparticle BUT almost 80% could evaluate the information given in the passage

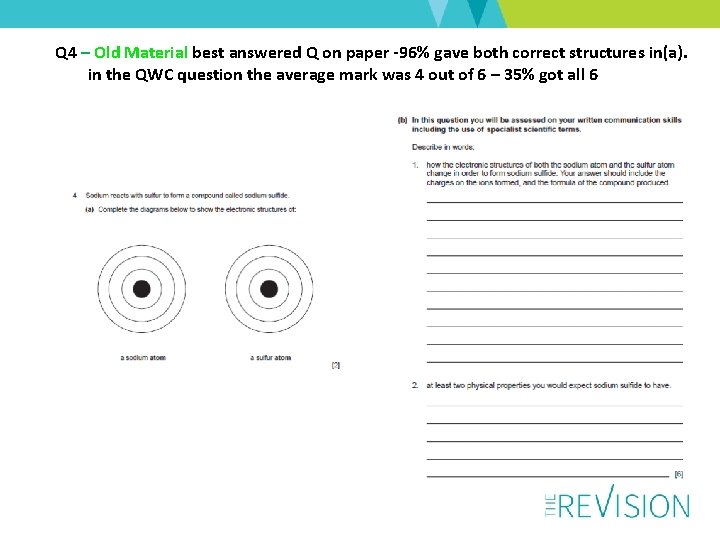
Q 4 – Old Material best answered Q on paper -96% gave both correct structures in(a). in the QWC question the average mark was 4 out of 6 – 35% got all 6

Q 5 – Old and new Material -2 nd best answered Q on paper. In (a) about 35% got 5/5 and the average mark was 3. 5; In (b) about 60% scored 3/3 but 10% scored 0

Q 6 – Old and new Material –Definition of relative atomic mass was very poorly recalled -54% scored 0/3 and only 21% scored 3/3; The first three calculations, which were all from the old spec were brilliantly answered about 88% correct overall BUT part ( c )(ii) (NEW material) answers seemed to be centre specific – 33% scored 0/3 - and 50% scored 3/3

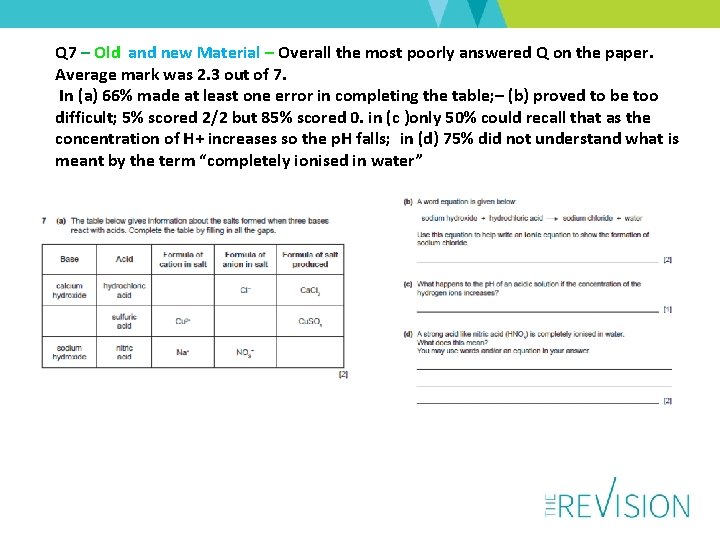
Q 7 – Old and new Material – Overall the most poorly answered Q on the paper. Average mark was 2. 3 out of 7. In (a) 66% made at least one error in completing the table; – (b) proved to be too difficult; 5% scored 2/2 but 85% scored 0. in (c )only 50% could recall that as the concentration of H+ increases so the p. H falls; in (d) 75% did not understand what is meant by the term “completely ionised in water”

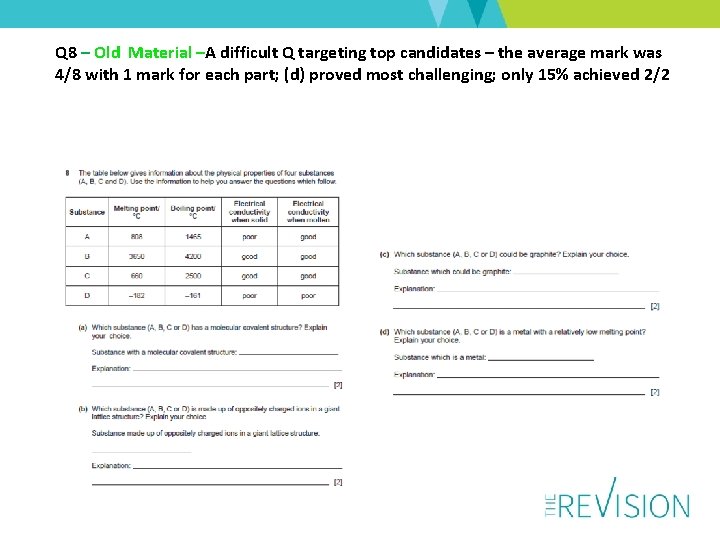
Q 8 – Old Material –A difficult Q targeting top candidates – the average mark was 4/8 with 1 mark for each part; (d) proved most challenging; only 15% achieved 2/2

Q 9 – New Material –In (a) answers tended to be centre specific – 35% scored 0/2 in the calculation but 60% gained both marks; (b) was very poorly answered- 70% scored 0/2 and very, very few scored 2

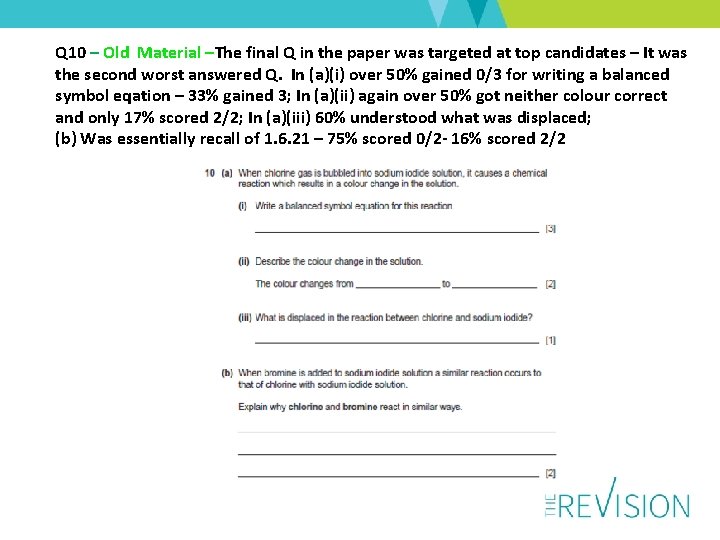
Q 10 – Old Material –The final Q in the paper was targeted at top candidates – It was the second worst answered Q. In (a)(i) over 50% gained 0/3 for writing a balanced symbol eqation – 33% gained 3; In (a)(ii) again over 50% got neither colour correct and only 17% scored 2/2; In (a)(iii) 60% understood what was displaced; (b) Was essentially recall of 1. 6. 21 – 75% scored 0/2 - 16% scored 2/2


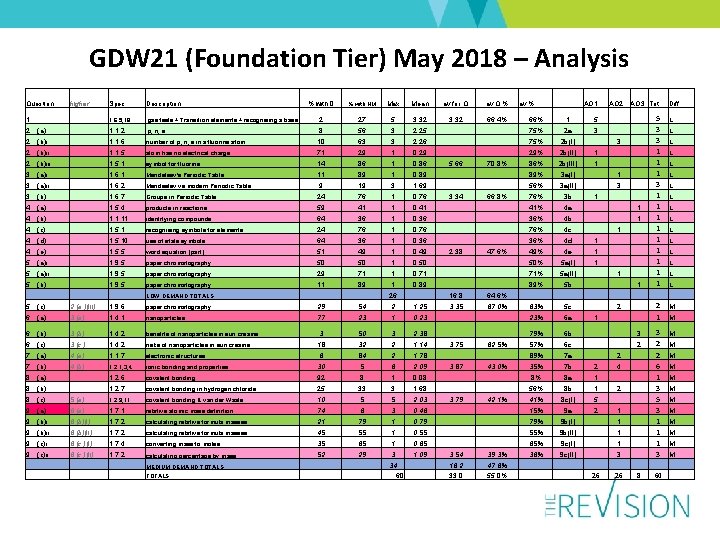
GDW 21 (Foundation Tier) May 2018 – Analysis Question higher Spec Description % with 0 % with HM Max Mean av for Q av Q % 1 1. 6. 5, 18 2 (a) 1. 1. 2 2 (b)i 1. 1. 6 2 (b)ii gas tests + Transition elements + recognising a base 2 27 5 3. 32 66. 4% 66% 1 5 p, n, e 8 56 3 2. 25 75% 2 a 3 number of p, n, e in a fluorine atom 10 63 3 2. 26 75% 2 b(i) 3 1. 1. 5 atom has no electrical charge 71 29 1 0. 29 29% 2 b(ii) 1 2 (b)iii 1. 5. 1 symbol for fluorine 14 86 1 0. 86 5. 66 70. 8% 86% 2 b(iii) 1 3 (a)i 1. 6. 1 Mendeleev's Periodic Table 11 89 1 0. 89 89% 3 a(i) 1 3 (a)ii 1. 6. 2 Mendeelev vs modern Periodic Table 9 19 3 1. 69 56% 3 a(ii) 3 3 (b) 1. 6. 7 Groups in Periodic Table 24 76 1 0. 76 3. 34 66. 8% 76% 3 b 1 4 (a) 1. 5. 4 products in reactions 59 41 1 0. 41 41% 4 a 1 4 (b) 1. 1. 11 identifying compounds 64 36 1 0. 36 36% 4 b 1 4 (c) 1. 5. 1 recognising symbols for elements 24 76 1 0. 76 76% 4 c 1 4 (d) 1. 5. 10 use of state symbols 64 36 1 0. 36 36% 4 d 1 4 (e) 1. 5. 5 word equation (part) 51 49 1 0. 49 2. 38 47. 6% 49% 4 e 1 5 (a)i 1. 9. 5 paper chromatography 50 50 1 0. 50 50% 5 a(i) 1 5 (a)ii 1. 9. 5 paper chromatography 29 71 1 0. 71 71% 5 a(ii) 1 5 (b) 1. 9. 5 paper chromatography 11 89 1 0. 89 89% 5 b 1 LOW DEMAND TOTALS 26 16. 8 64. 6% 5 (c) 2 (a )(iii) 1. 9. 6 paper chromatography 29 54 2 1. 25 3. 35 67. 0% 63% 5 c 2 6 (a) 3 (a) 1. 4. 1 nanoparticles 77 23 1 0. 23 23% 6 a 1 6 (b) 3 (b) 1. 4. 2 benefits of nanoparticles in sun creams 3 50 3 2. 38 79% 6 b 3 6 (c) 3 (c ) 1. 4. 2 risks of nanoparticles in sun creams 18 32 2 1. 14 3. 75 62. 5% 57% 6 c 2 7 (a) 4 (a) 1. 1. 7 electronic structures 6 84 2 1. 78 89% 7 a 2 7 (b) 4 (b) 1. 2. 1, 3, 4 ionic bonding and properties 30 5 6 2. 09 3. 87 43. 0% 35% 7 b 2 4 8 (a) 1. 2. 6 covalent bonding 92 8 1 0. 08 8% 8 a 1 8 (b) 1. 2. 7 covalent bonding in hydrogen chloride 25 33 3 1. 68 56% 8 b 1 2 8 (c) 5 (a) 1. 2. 9, 11 covalent bonding & van der Waals 10 5 5 2. 03 3. 79 42. 1% 41% 8 c(i) 5 9 (a) 6 (a) 1. 7. 1 relative atomic mass definition 74 6 3 0. 46 15% 9 a 2 1 9 (b)i 6 (b)(i) 1. 7. 2 calculating relative formula masses 21 79 1 0. 79 79% 9 b(i) 1 9 (b)ii 6 (b)(ii) 1. 7. 2 calculating relative formula masses 45 55 1 0. 55 55% 9 b(ii) 9 (c)i 6 (c )(i) 1. 7. 4 converting mass to moles 35 65 1 0. 65 65% 9 c(i) 1 9 (c)ii 6 (c )(ii) 1. 7. 2 calculating percentage by mass 52 29 3 1. 09 3. 54 39. 3% 36% 9 c(ii) 3 MEDIUM DEMAND TOTALS 16. 2 33. 0 47. 6% 55. 0% 26 8 TOTALS 34 60 av % AO 1 AO 2 1 AO 3 Tot Diff 5 3 3 1 1 1 2 1 L 3 2 2 6 1 3 5 3 1 1 1 3 M L L L L M M M M 60

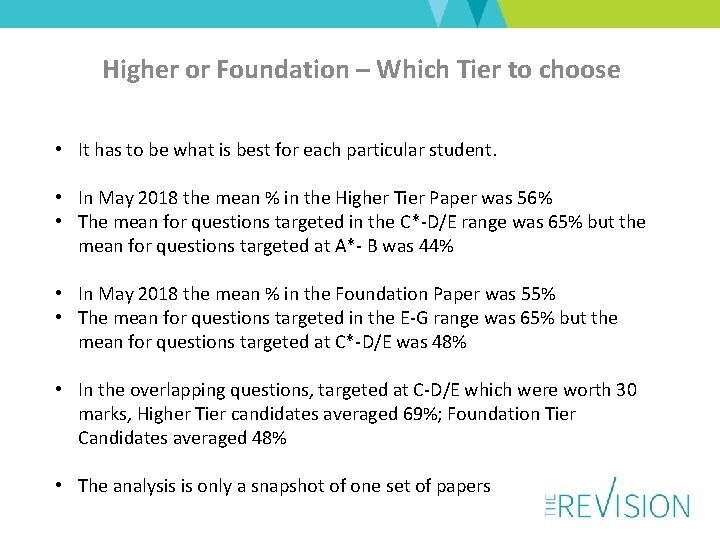
Higher or Foundation – Which Tier to choose • It has to be what is best for each particular student. • In May 2018 the mean % in the Higher Tier Paper was 56% • The mean for questions targeted in the C*-D/E range was 65% but the mean for questions targeted at A*- B was 44% • In May 2018 the mean % in the Foundation Paper was 55% • The mean for questions targeted in the E-G range was 65% but the mean for questions targeted at C*-D/E was 48% • In the overlapping questions, targeted at C-D/E which were worth 30 marks, Higher Tier candidates averaged 69%; Foundation Tier Candidates averaged 48% • The analysis is only a snapshot of one set of papers

GCSE Chemistry: Unit 3 GCSE DAS Chemistry: Unit 7 THE PRACTICAL SKILLS UNIT

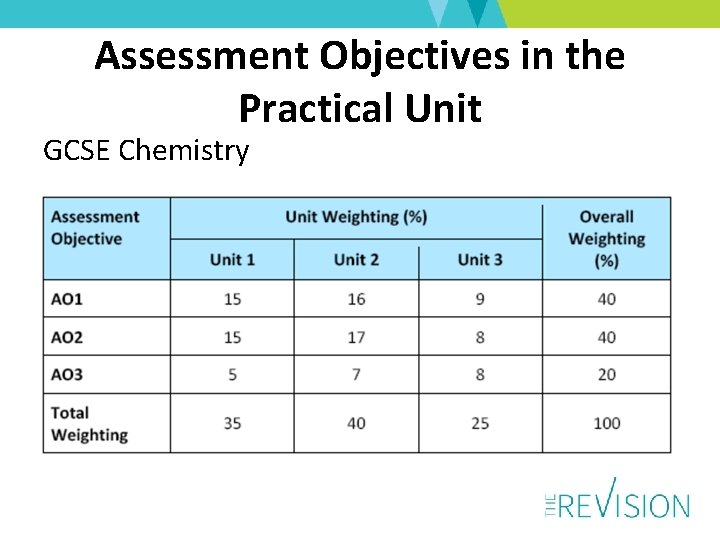
Assessment Objectives in the Practical Unit GCSE Chemistry

Assessment Objectives in the Practical Unit GCSE DAS: Chemistry

Changes from CATs • Booklet A and B is synoptic • Reduced AO 3 weighting in this unit • A significant amount of AO 1 now in this unit (mostly in Booklet B) • Booklet B – Emphasis on transferable practical skills – Links knowledge to practical work • Practical Manuals are support materials but not exact examination methods

General Information • Tier of entry – Same Tier for whole unit (Booklet A and B and all disciplines) – Cannot be changed after first Booklet A is sat • Format of papers – Paper marking for Booklet A (FT: Buff and HT White) – Online marking for Booklet B • External assessment – Marked by CCEA – CCEA will collect Booklet A on the first Friday in June – Booklet A will be marked by Centre so the mark scheme can be adapted to account for Centre Issues – report must be emailed to the Subject Officer

• • • How they will be marked? – Booklet A and B will be marked by two separate examining teams and the marks will be aggregated – DAS Booklet A’s (Bio, Chem and Phys) will be marked by a generic Science marking team – DAS Booklet B’s will be marked by three separate subject specialist teams Resits – the whole unit must be resat – Can be resat at a different tier – Only offered in the summer series Review of marking – GCSE Chemistry: Booklet A and B can be remarked individually and will have two individual applications and charges – GCSE DAS: Booklet A, Biology Booklet B, Chemistry Booklet B and Physics Booklet B can be remarked individually by choice and will have four individual applications and charges – Each component within the unit will have results reports individually as well as at unit level

Skills Assessed in Booklet A • Planning techniques – identifying variables, drawing and choosing appropriate apparatus • Risk Assessment of a given method or chemical • Recording observations • Recording measurements with units • Drawing or using a suitable for recording results • Processing of results/observations including mathematical processing • Graph plotting and line of best fit

Skills Assessed in Booklet B • The objective of Booklet B to assess practical theory, deductions and calculations in familiar and unfamiliar contexts. • Assessment may include the following: – Planning techniques – identifying variables, drawing and choosing appropriate apparatus – risk assessment – analysis/processing of secondary data – drawing graphs – Identifying trends – Reviewing scientific methodology and apparatus – Making conclusions

Alyn Mc. Farland GCSE CHEMISTRY

Emphasis on Practical Work • 9 Prescribed practicals • Other practical opportunities in the specification • 100 out of the 280 raw marks at Higher Tier for unit 3 practical chemistry • Practical questions in Units 1 and 2 • Synoptic nature of Unit 3

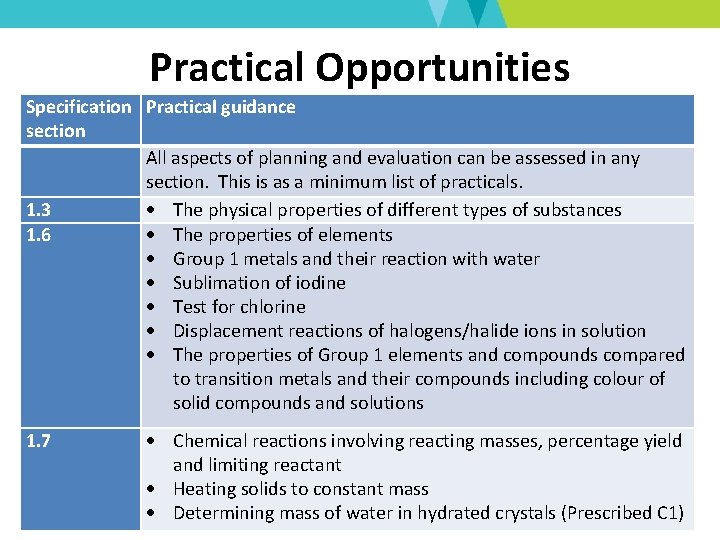
Practical Opportunities Specification Practical guidance section All aspects of planning and evaluation can be assessed in any section. This is as a minimum list of practicals. 1. 3 The physical properties of different types of substances 1. 6 The properties of elements Group 1 metals and their reaction with water Sublimation of iodine Test for chlorine Displacement reactions of halogens/halide ions in solution The properties of Group 1 elements and compounds compared to transition metals and their compounds including colour of solid compounds and solutions 1. 7 Chemical reactions involving reacting masses, percentage yield and limiting reactant Heating solids to constant mass Determining mass of water in hydrated crystals (Prescribed C 1)

Practical Opportunities Specification Practical guidance section 1. 8 Indicators and p. H meter Reactions of acids including temperature changes (Prescribed C 2) Tests for CO 2 and H 2 gases Salt preparations (Prescribed C 3) 1. 9 Separating techniques (filtration, crystallisation, paper chromatography, simple distillation and fractional distillation) Test for water Tests for ions (Prescribed C 4) 1. 10 Determination of solubility (other practical activity) Determination of mass of solid deposited on cooling a solution 2. 1 Reactions of metals with air, water and steam and displacement reactions (Prescribed C 5) Reactions with an unfamiliar metal

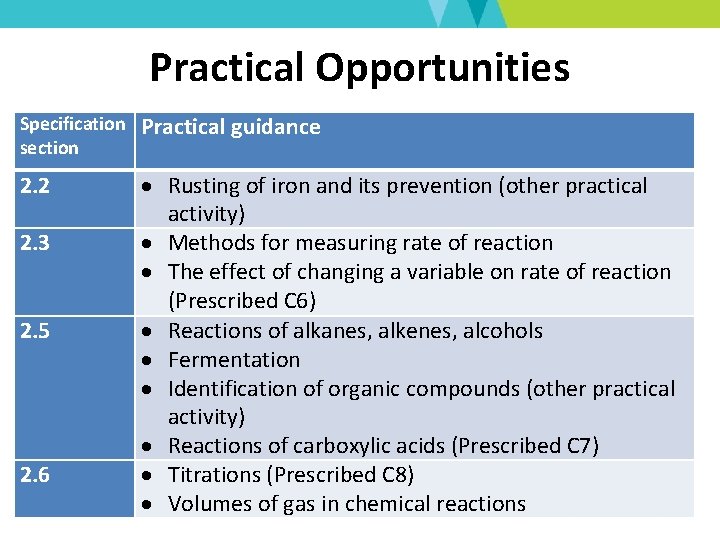
Practical Opportunities Specification section 2. 2 2. 3 2. 5 2. 6 Practical guidance Rusting of iron and its prevention (other practical activity) Methods for measuring rate of reaction The effect of changing a variable on rate of reaction (Prescribed C 6) Reactions of alkanes, alkenes, alcohols Fermentation Identification of organic compounds (other practical activity) Reactions of carboxylic acids (Prescribed C 7) Titrations (Prescribed C 8) Volumes of gas in chemical reactions

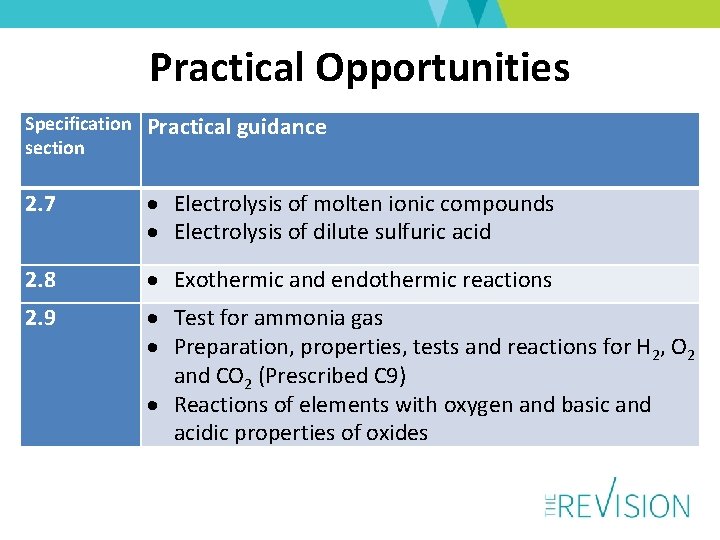
Practical Opportunities Specification section Practical guidance 2. 7 Electrolysis of molten ionic compounds Electrolysis of dilute sulfuric acid 2. 8 Exothermic and endothermic reactions 2. 9 Test for ammonia gas Preparation, properties, tests and reactions for H 2, O 2 and CO 2 (Prescribed C 9) Reactions of elements with oxygen and basic and acidic properties of oxides

Practical Skills • Observables, skills and processing – Quality of observations – Drawing apparatus – Recording numerical values with units – Significant figures, decimal places and rounding – Tables – Graphs

Observations • Quality of observations – “heat and bubbles” – Some observations given and others asked – Choose from a list of observations – Observations given and chemistry questioned – Incorrect observations “CON” symbol used

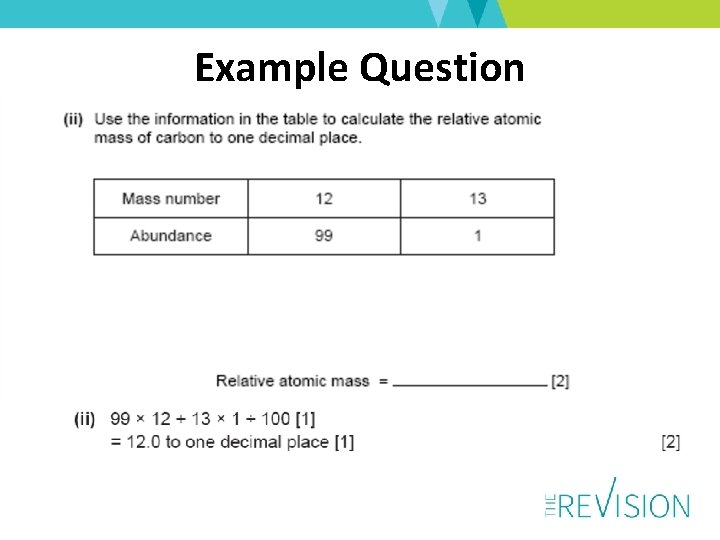
Example Question

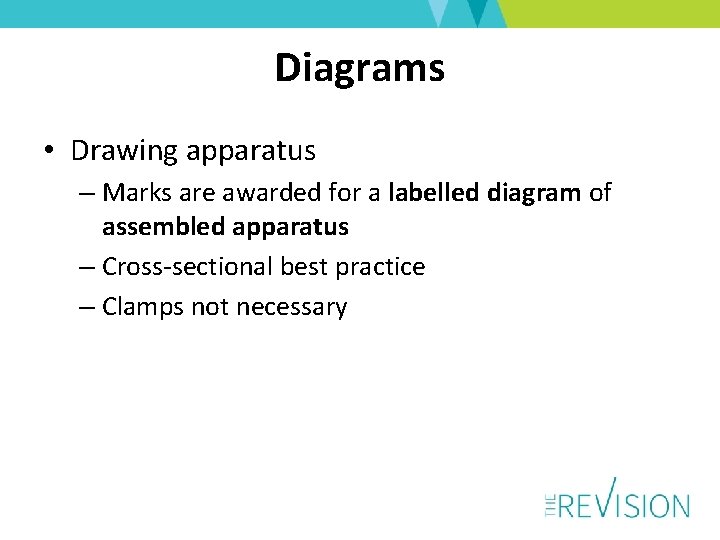
Diagrams • Drawing apparatus – Marks are awarded for a labelled diagram of assembled apparatus – Cross-sectional best practice – Clamps not necessary

Example Question

Recording Numerical Data • Recording numerical data with units – Units given in calculations at end of line – Where processing required, units may be required (may be Tier dependent) – Incorrect unit would be penalised


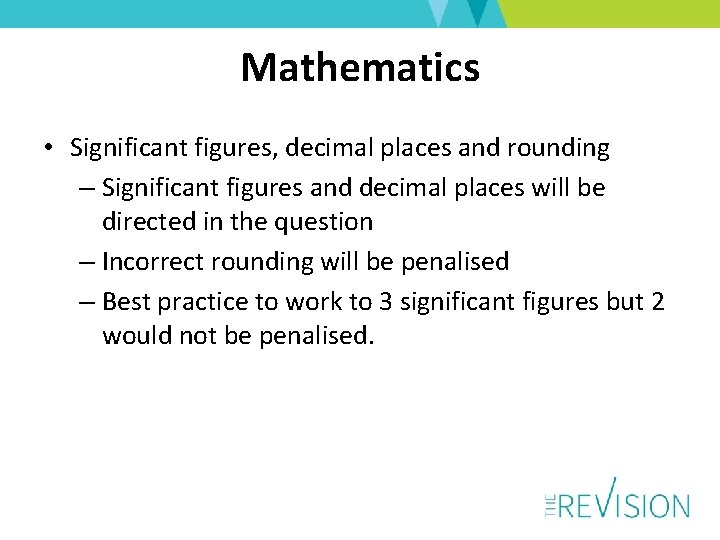
Mathematics • Significant figures, decimal places and rounding – Significant figures and decimal places will be directed in the question – Incorrect rounding will be penalised – Best practice to work to 3 significant figures but 2 would not be penalised.

Example Question

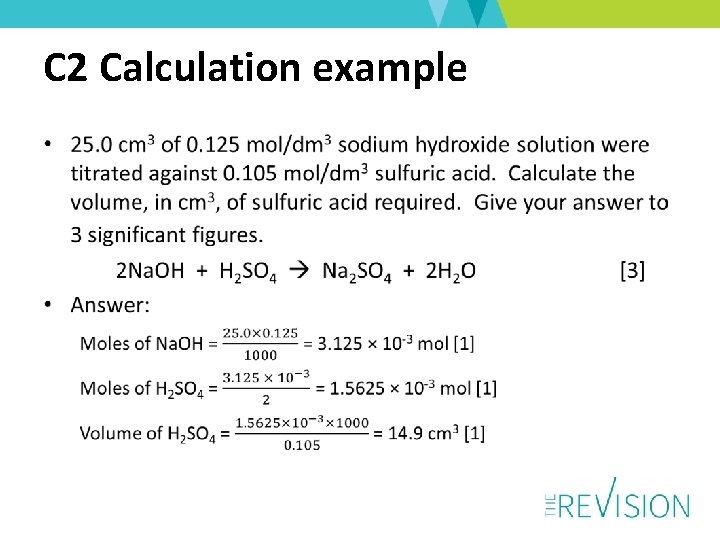
Calculation example • Calculate the mass of copper(II) oxide formed when 3. 33 g of copper(II) carbonate are heated to constant mass. Cu. CO 3 Cu. O + CO 2 [3] • Answer: Moles of copper(II) carbonate = = 0. 0269 mol [1] Moles of copper(II) oxide = 0. 0269 mol [1] Mass of copper(II) oxide = 0. 0269 × 80 = 2. 15 g [1]

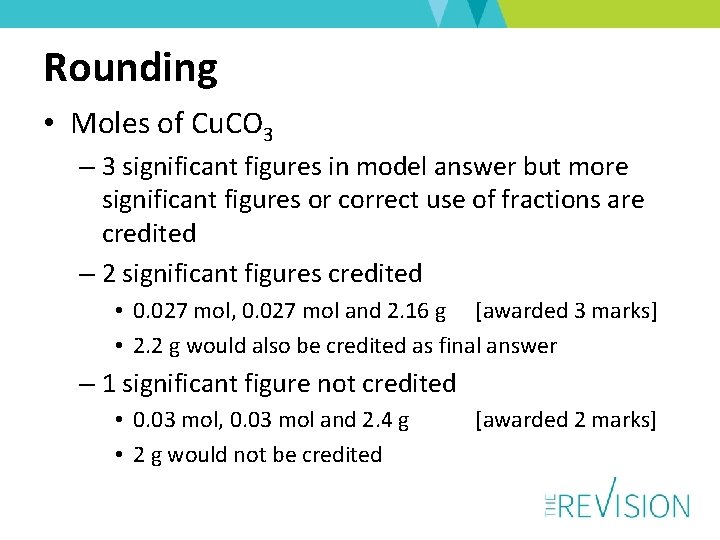
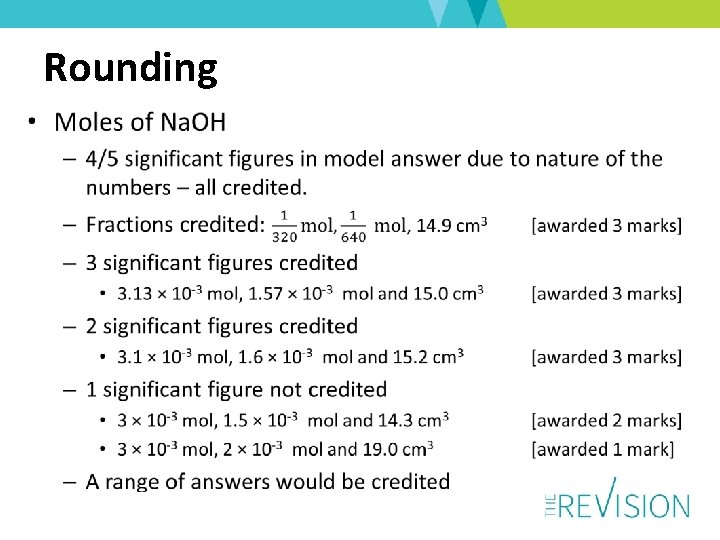
Rounding • Moles of Cu. CO 3 – 3 significant figures in model answer but more significant figures or correct use of fractions are credited – 2 significant figures credited • 0. 027 mol, 0. 027 mol and 2. 16 g [awarded 3 marks] • 2. 2 g would also be credited as final answer – 1 significant figure not credited • 0. 03 mol, 0. 03 mol and 2. 4 g • 2 g would not be credited [awarded 2 marks]

C 2 Calculation example •

Rounding •

Tables • Tables – Best practice is a complete boxed table with headings and units – Units should not be in the body of the table – Numbers recorded to the same number of decimal places in the table, where appropriate

Emphasis on practical work

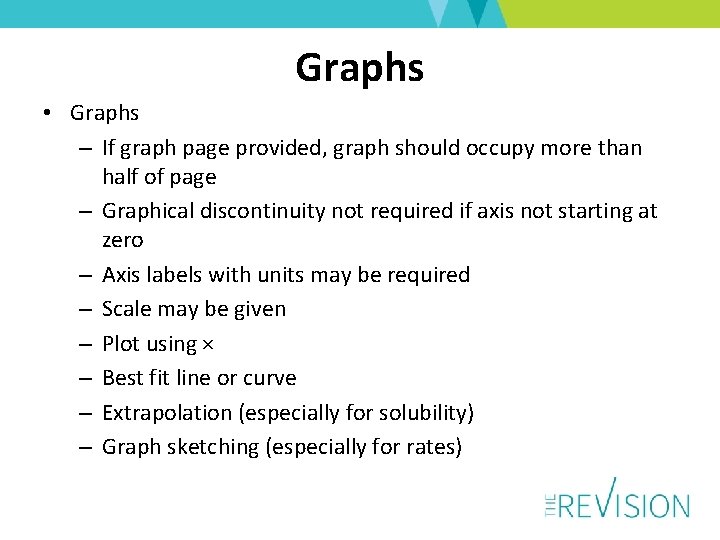
Graphs • Graphs – If graph page provided, graph should occupy more than half of page – Graphical discontinuity not required if axis not starting at zero – Axis labels with units may be required – Scale may be given – Plot using × – Best fit line or curve – Extrapolation (especially for solubility) – Graph sketching (especially for rates)



Bob Cummings GCSE DAS: CHEMISTRY

Practical Opportunities Specification Practical guidance section All aspects of planning and evaluation can be assessed in any section. This is as a minimum list of practicals. 1. 3 The physical properties of different types of substances 1. 6 1. 7 Chemical reactions involving reacting masses, percentage yield and limiting reactant The properties of elements Group 1 metals and their reaction with water Sublimation of iodine Test for chlorine Displacement reactions of halogens/halide ions in solution The properties of Group 1 elements and compounds including colour of solid compounds and solutions

Practical Opportunities Specification Practical guidance section 1. 8 1. 9 Separating techniques (filtration, crystallisation, paper chromatography, simple distillation and fractional distillation) Test for water Flame Tests (Prescribed C 2) Indicators and p. H meter Reactions of acids including temperature changes (Prescribed C 1) Tests for CO 2 and H 2 gases Colour of Group 2, aluminium and zinc salts and solutions

Practical Opportunities Specification section 2. 1 2. 2 Practical guidance Reactions of metals with air, water and steam and displacement reactions (Prescribed C 3) Reactions of unfamiliar elements Rusting of iron & its prevention (other practical activity) 2. 3 Methods for measuring rate of reaction The effect of changing a variable on rate of reaction (Prescribed C 4) including particle size (other practical activity) 2. 5 Reactions of alkanes, alkenes, alcohols Determining the presence of a C=C using bromine water Fermentation Identification of organic compounds

Practical Opportunities Specification section Practical guidance 2. 6 2. 7 Heating solids to constant mass Determining the mass of water in hydrated crystals (Prescribed C 5) Electrolysis of molten ionic compounds 2. 8 Exothermic and endothermic reactions 2. 9 Test for ammonia gas Preparation, properties, tests and reactions for H 2, O 2 and CO 2 (Prescribed C 6) Reactions of elements with oxygen and basic and acidic properties of oxides

A Little quiz • In the May 2018 GDW 22 paper how many of the 70 marks related to practical activity 5 10 15 20 25 or 30 ? • What was the average percentage of those marks gained by candidates? 70% 60% 50% 40% 30% or 20% • 57% 23% 55% 82% 36% 19% 30% 15% 52% 54% 42% What do these percentages represent? • 43% 23% 45% 18% 64% 74% 67% 34% 33% 17% 58% And what do these percentages represent?

Preparing for the Unit 2 & Practical B exam day • In both papers, students who have had significant practical opportunities will be at an advantage • This does not mean that students have to “have done” every possible practical • It does mean that students need to have observed a wide range of practical activities • “bubbles of gas”, “heat given off”, wear goggles etc. are unlikely to form a good basis for high marks • Although there are 150 marks available across DAS for the Practical Assessment, the Tier of the first 15 marks (i. e. the first Booklet A paper taken) determines the Tier for the other 135 marks across the other 5 practical papers

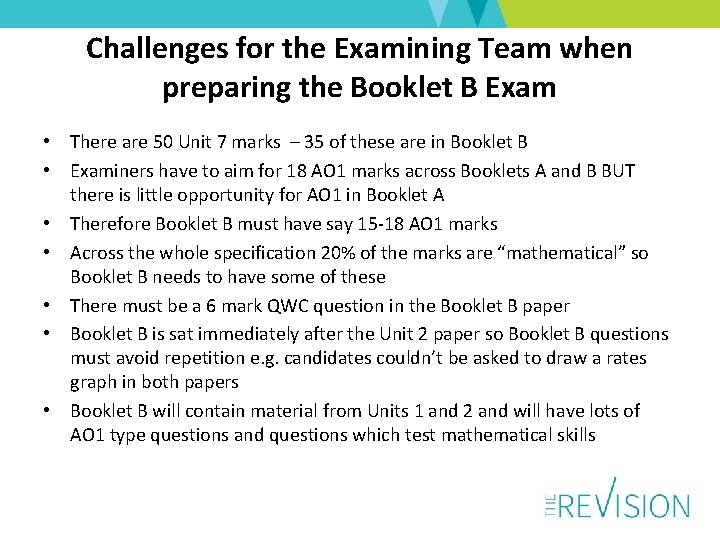
Challenges for the Examining Team when preparing the Booklet B Exam • There are 50 Unit 7 marks – 35 of these are in Booklet B • Examiners have to aim for 18 AO 1 marks across Booklets A and B BUT there is little opportunity for AO 1 in Booklet A • Therefore Booklet B must have say 15 -18 AO 1 marks • Across the whole specification 20% of the marks are “mathematical” so Booklet B needs to have some of these • There must be a 6 mark QWC question in the Booklet B paper • Booklet B is sat immediately after the Unit 2 paper so Booklet B questions must avoid repetition e. g. candidates couldn’t be asked to draw a rates graph in both papers • Booklet B will contain material from Units 1 and 2 and will have lots of AO 1 type questions and questions which test mathematical skills

Q&A ?

Contact Details Education Manager: Elaine Lennox Telephone: 028 90 261200 Ext. 2320 Email: elennox@ccea. org. uk Subject Support Officer: Nuala Tierney Telephone: 028 90 261200 Ext. 2292 Email: ntierney@ccea. org. uk Specification, sample assessment and support materials available on the subject microsite at www. ccea. org. uk

Subscriber Campaign CCEA are moving to more email based communications with all our centres and this will include providing teachers with - • information on subject updates • information on subject events • information on new support materials • general news information It’s important that YOU subscribe using the link on the homepage of our website or http: //ccea. org. uk/signup

http: //ccea. org. uk/signup

Working with CCEA The benefits: – teacher cover provided – first-hand experience of how the examining system works – user insight to the standards required for the assessment – opportunity to examine assessments across a range of abilities – improved learning and teaching outcomes – creates links with CCEA personnel/subject officer – opportunity to network with other professionals – provides recognition and enhances the professional development of teachers

Why choose CCEA? We support Learners - CCEA puts the learner at the centre of everything we do. We think about what learners need for life and work and then build solutions to meet those needs. We do this for the entire curriculum – from Foundation and Early Years to A level and beyond. We are Local - CCEA is Northern Ireland’s awarding body. We understand local needs and we are focused on providing services and products for learners in Northern Ireland. This also means we’re near you, should you need help or support. We are Listening - CCEA listens to those who use its products and services; this means listening to teachers, employers and learners, and taking action to ensure better outcomes for learners. This approach ensures that we develop relevant, high quality and innovative specifications and support.
 Mutually exclusive vs non mutually exclusive
Mutually exclusive vs non mutually exclusive Probability foundation gcse questions
Probability foundation gcse questions Das alte ist vergangen das neue angefangen
Das alte ist vergangen das neue angefangen Eu fico com.a pureza
Eu fico com.a pureza Das alles ist deutschland das alles sind wir
Das alles ist deutschland das alles sind wir Jesus sagt ich bin das licht der welt
Jesus sagt ich bin das licht der welt O menosprezo das artes e das letras
O menosprezo das artes e das letras Pixl knowit gcse chemistry quantitative
Pixl knowit gcse chemistry quantitative Aqa chemistry gcse required practicals
Aqa chemistry gcse required practicals Example of major point
Example of major point Salters advanced chemistry
Salters advanced chemistry Ccea gce chemistry practical support
Ccea gce chemistry practical support Functional groups ib chemistry
Functional groups ib chemistry Inorganic chemistry vs organic chemistry
Inorganic chemistry vs organic chemistry Who fought in the punic wars?
Who fought in the punic wars? What happened sunday morning in romeo and juliet
What happened sunday morning in romeo and juliet Identify independent and dependent events
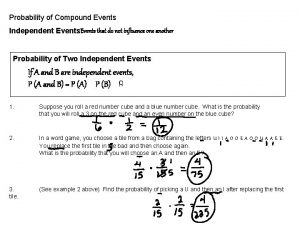
Identify independent and dependent events Simple and compound events examples
Simple and compound events examples Independent event in probability
Independent event in probability It looks for patterns recurring characteristics and events
It looks for patterns recurring characteristics and events 7-3 independent and dependent events answers
7-3 independent and dependent events answers Exhaustive events
Exhaustive events Disadvantages of gagne's instructional events
Disadvantages of gagne's instructional events Grandfather clause apush
Grandfather clause apush Cellular events of acute inflammation
Cellular events of acute inflammation Intersection and conditional probability
Intersection and conditional probability Disjoint event example
Disjoint event example What are the short story
What are the short story Time clocks and the ordering of events
Time clocks and the ordering of events What role do automobiles play in the great gatsby
What role do automobiles play in the great gatsby Expressive speech act
Expressive speech act National and kapodistrian university of athens events
National and kapodistrian university of athens events Leslie lamport time clocks
Leslie lamport time clocks Safety at sports and recreational events act
Safety at sports and recreational events act Mutually exclusive examples
Mutually exclusive examples Elements of the setting
Elements of the setting Time clocks and the ordering of events
Time clocks and the ordering of events Written and other recorded events of people
Written and other recorded events of people P(a and b) formula
P(a and b) formula 10-7 lesson quiz
10-7 lesson quiz Significant life events health and social care
Significant life events health and social care Probability of overlapping events
Probability of overlapping events Sample space and events
Sample space and events Sample outcome
Sample outcome Brainpop independent and dependent events answers
Brainpop independent and dependent events answers 13-5 probabilities of independent and dependent events
13-5 probabilities of independent and dependent events Describing people and events
Describing people and events Sedimentary rocks record past geological events and ____.
Sedimentary rocks record past geological events and ____. Setting events and antecedents
Setting events and antecedents Sample design and technology gcse examination paper answers
Sample design and technology gcse examination paper answers Sample design and technology gcse examination paper answers
Sample design and technology gcse examination paper answers Www.technologystudent.com
Www.technologystudent.com Gcse
Gcse Reverse mean problems
Reverse mean problems Edexcel gcse art and design
Edexcel gcse art and design Here is a solid square based pyramid vabcd
Here is a solid square based pyramid vabcd Fractions decimals and percentages gcse
Fractions decimals and percentages gcse Ocr product design gcse
Ocr product design gcse Advantages and disadvantages of nanoparticles gcse
Advantages and disadvantages of nanoparticles gcse Between a rock and a hard place gcse
Between a rock and a hard place gcse Edexcel gcse history crime and punishment exam questions
Edexcel gcse history crime and punishment exam questions Gcse business and communication systems
Gcse business and communication systems Gcse history questions and answers
Gcse history questions and answers Sample design and technology gcse examination paper answers
Sample design and technology gcse examination paper answers Factors multiples and primes worksheet gcse
Factors multiples and primes worksheet gcse Solenoid bbc bitesize
Solenoid bbc bitesize Jekyll and hyde key quotes
Jekyll and hyde key quotes Hcf lcm gcse questions
Hcf lcm gcse questions Unit 2 leisure time
Unit 2 leisure time Error intervals gcse questions and answers
Error intervals gcse questions and answers Area and perimeter gcse exam questions higher
Area and perimeter gcse exam questions higher Aqa art gcse
Aqa art gcse Gcse leisure and tourism
Gcse leisure and tourism Private limited company gcse business
Private limited company gcse business Similar shapes area and volume gcse questions
Similar shapes area and volume gcse questions Gcse design and technology coursework examples 2019
Gcse design and technology coursework examples 2019 Gcse business and communication systems
Gcse business and communication systems Are sole traders in the private sector
Are sole traders in the private sector Tree diagram with replacement example
Tree diagram with replacement example Put the following events in chronological order
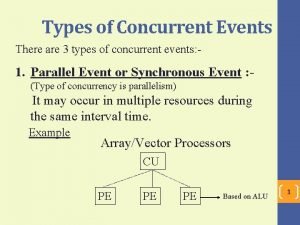
Put the following events in chronological order Concurrent events examples
Concurrent events examples Tkam chapter 12-15 summary
Tkam chapter 12-15 summary Martin luther king timeline of events
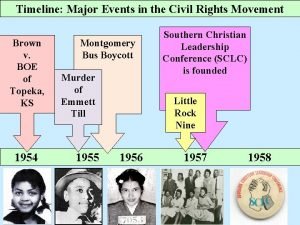
Martin luther king timeline of events Timeline of good friday events
Timeline of good friday events Civil rights timeline of events
Civil rights timeline of events Painting
Painting Things fall apart sparknotes 17-19
Things fall apart sparknotes 17-19 Renaissance characteristics
Renaissance characteristics What years did the renaissance take place
What years did the renaissance take place Summary of the renaissance
Summary of the renaissance The plot sequence
The plot sequence War with austria french revolution
War with austria french revolution The crucible timeline of important events
The crucible timeline of important events Independent probability formula
Independent probability formula Near miss adverse event sentinel event
Near miss adverse event sentinel event Scentsy sales tax
Scentsy sales tax Risk breakdown structure
Risk breakdown structure Adverse events in hospital
Adverse events in hospital Ct dph reportable event classifications
Ct dph reportable event classifications Secular music
Secular music How did the writer feel about this experience
How did the writer feel about this experience Adverse events in hospital
Adverse events in hospital Put the following events into the correct order.
Put the following events into the correct order. Puerperal sepsis definition
Puerperal sepsis definition Independent probability
Independent probability A tree diagram with replacement is for independent events.
A tree diagram with replacement is for independent events. Combined events probability
Combined events probability Disjoint events
Disjoint events Collectively exhaustive examples
Collectively exhaustive examples Never events liste
Never events liste Post ww2 events
Post ww2 events Subsequent event dan contohnya
Subsequent event dan contohnya Tujuan pemeriksaan subsequent events
Tujuan pemeriksaan subsequent events Panther involvement network
Panther involvement network Venn diagrams mutually exclusive
Venn diagrams mutually exclusive Hyperledger fabric events
Hyperledger fabric events Sequence of events at the neuromuscular junction
Sequence of events at the neuromuscular junction Is a spoken or written account of connected events
Is a spoken or written account of connected events A series of unfortunate events michael wolf
A series of unfortunate events michael wolf Discrepant event examples
Discrepant event examples