COMPLEX IONS CHEM 112 COMPLEX Ions Complex metal












![EXAMPLE: [Ni(NH 3)6]2+ Name: hexamine nickel (II) ion Coordination number : 6 Metal oxidation EXAMPLE: [Ni(NH 3)6]2+ Name: hexamine nickel (II) ion Coordination number : 6 Metal oxidation](https://slidetodoc.com/presentation_image_h2/2bf3fe521400395b3dba88afdbb9ee0d/image-19.jpg)
![EXAMPLE: [Co. Cl 2(en)2]+ Name : dichlorobis(ethylenediamine)cobalt III Coordination number : 6 (en is EXAMPLE: [Co. Cl 2(en)2]+ Name : dichlorobis(ethylenediamine)cobalt III Coordination number : 6 (en is](https://slidetodoc.com/presentation_image_h2/2bf3fe521400395b3dba88afdbb9ee0d/image-20.jpg)
![EXAMPLE: • Potassium amminetrichloroplatinate II Formula : K[Pt. Cl 3(NH 3)] Coordination number : EXAMPLE: • Potassium amminetrichloroplatinate II Formula : K[Pt. Cl 3(NH 3)] Coordination number :](https://slidetodoc.com/presentation_image_h2/2bf3fe521400395b3dba88afdbb9ee0d/image-21.jpg)
- Slides: 21

COMPLEX IONS CHEM 112

COMPLEX Ions Complex – metal atom or ion with attached groups called ligands

Transition metals commonly the complex ions contain transition metal compounds variable oxidation number colored unusual composition covalent compounds bonded to the metal coordinate covalent bonds unusual magnetic properties

ex: Cisplatin : diamminedichloridoplatinum II platinum complex binds to DNA causing the DNA to crosslink and die in a programmed way

Ex: Cisplatin once administered in IV at least one of the ligands is replaced by water to give the aquo complex: [Pt. Cl(NH 3)2(H 2 O)]+ the aquo ligand is displaced by guanine in the cell [Pt. Cl(guanine-DNA)(NH 3)2]+ this causes cross-linking and disrupts mitosis

COMPLEXES • Coordination sphere – the area of the central atom/ion and ligands • Coordination number- the number of points where the ligands attach

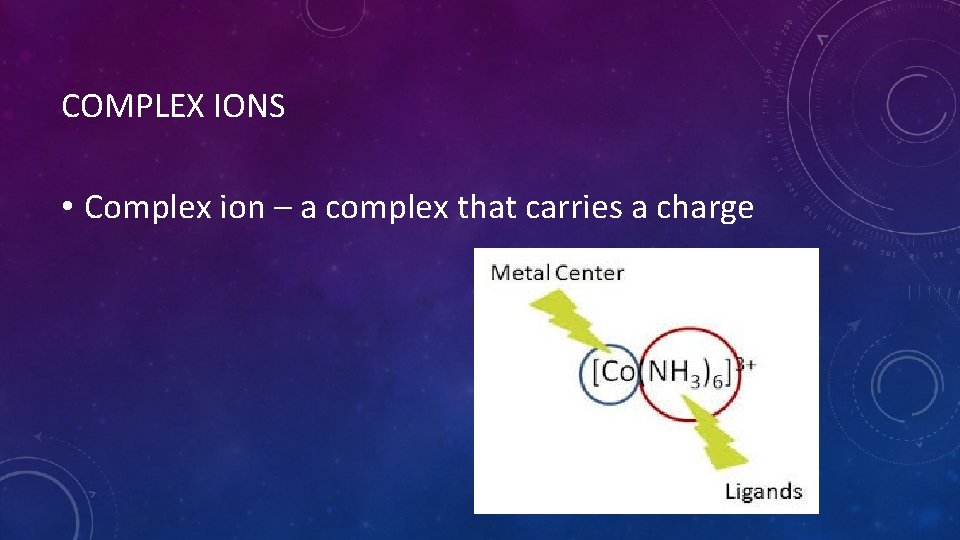
COMPLEX IONS • Complex ion – a complex that carries a charge

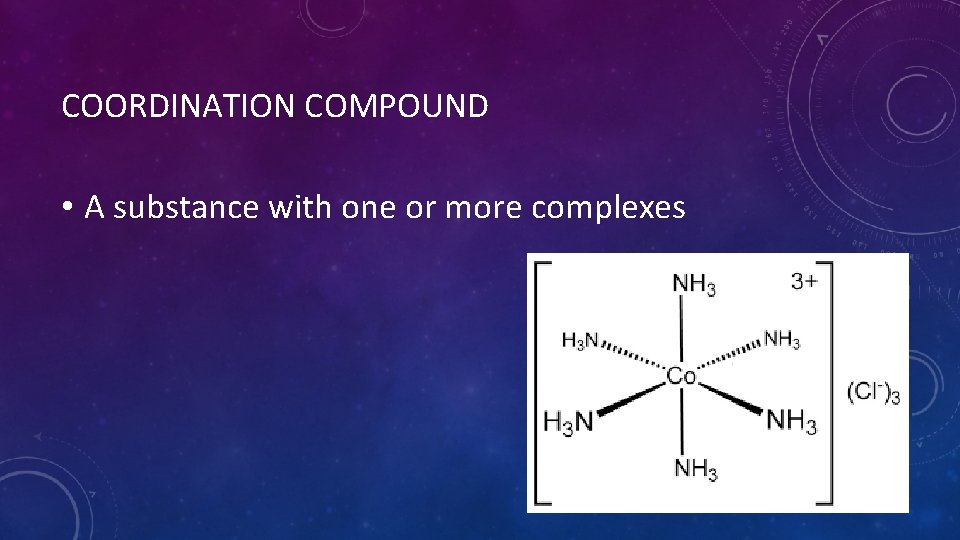
COORDINATION COMPOUND • A substance with one or more complexes


COMMON LIGANDS • CN cyano • NO 2 - nitro • NO nitrosyl • O 2 - oxo

LIGANDS FROM COORDINATE COVALENT BONDS • Transition metals have empty d-orbitals that can accept electron pairs • Ligands have electron pairs to donate Ligand type electron pairs donated Monodentate 1 Bidentate 2 Polydentate more than 1 Chelate 5 or 6

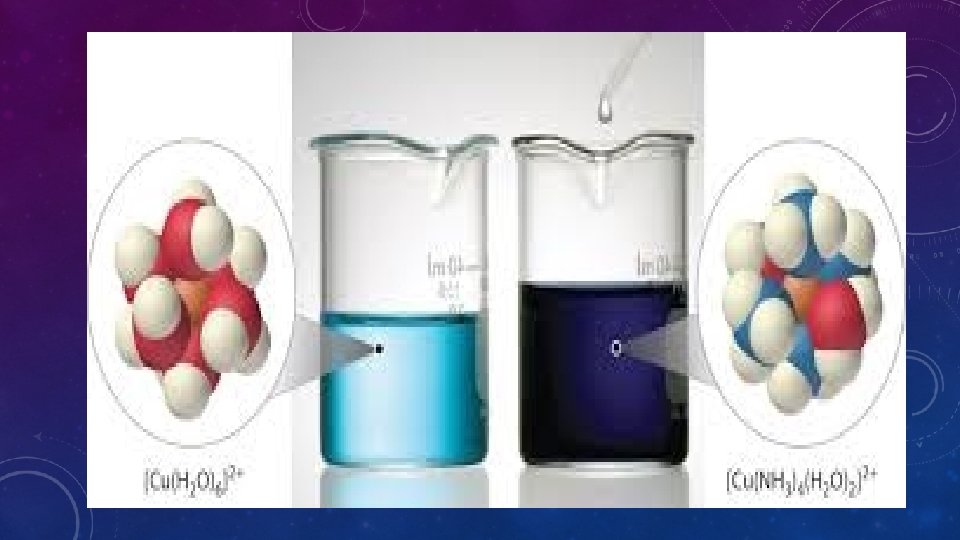
Chelates EDTA - tie up calcium ions in bathroom cleaners, shower sprays prevent blood clots to remove heavy metals from body when poisoned solubilize iron in plant fertilizer remove the iron taste from mayonnaise (prepared in iron vats)

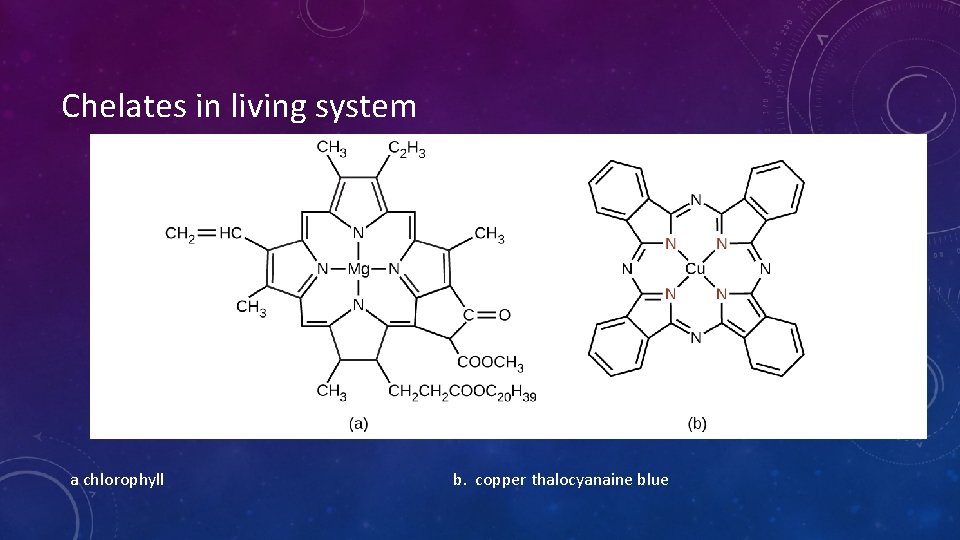
Chelates in living system a chlorophyll b. copper thalocyanaine blue

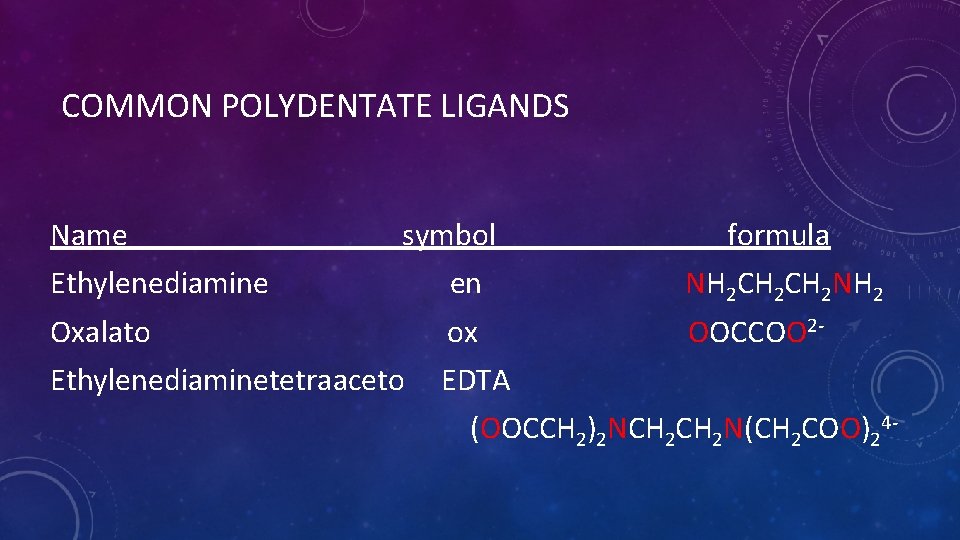
COMMON POLYDENTATE LIGANDS Name symbol formula Ethylenediamine en NH 2 CH 2 NH 2 Oxalato ox OOCCOO 2 - Ethylenediaminetetraaceto EDTA (OOCCH 2)2 NCH 2 N(CH 2 COO)24 -

NAMING COORDINATION COMPLEXES • 1. Name, as a single word, ligands in alphabetical order by name (ignoring Greek prefixes) then the central atom or ion of the complex • 2. Indicate the number of each ligand in a complex with a Greek prefix (di=2, tri=3, tera=4, etc. ). If the ligand name has a Greek prefix, place the parentheses around that name and prefix it with bis=2 pentakis = 5 tris = 3 hexakis = 6 tetrakis = 4

NAMING COORDINATION COMPLEXES • 3. Name complex cations with the name of the central metal. Name complex anions by adding the –ate to the end of the central metal (some metal anions have Latin based names – cuprate, ferrate, aurate, argentite, plumbate, stannate). Always put a Roman numeral in parentheses after the name of the metal to show its oxidation number • 4. For coordination compounds, name the cation the anion

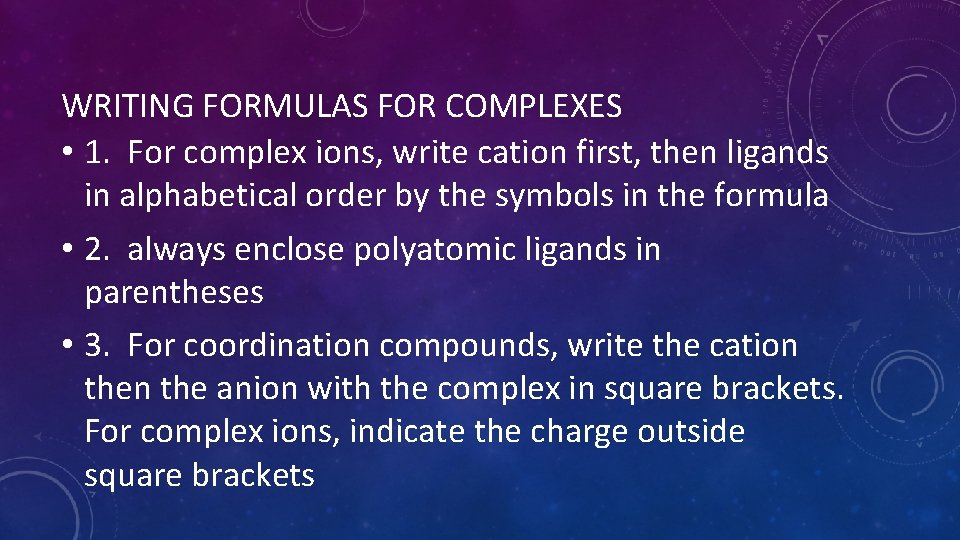
WRITING FORMULAS FOR COMPLEXES • 1. For complex ions, write cation first, then ligands in alphabetical order by the symbols in the formula • 2. always enclose polyatomic ligands in parentheses • 3. For coordination compounds, write the cation the anion with the complex in square brackets. For complex ions, indicate the charge outside square brackets

NAMING LIGANDS - ATTACHMENT POINT IN RED SEE CHART PG 854 Neutral ligands • • NH 3 amine H 2 O aqua CO carbonyl CH 3 NH 2 methylamine Anionic ligands • F- fluoro • Cl- chloro • Br- bromo • I- iodo • OH- hydroxo • CN- cyano • OSO 32 - sulfato
![EXAMPLE NiNH 362 Name hexamine nickel II ion Coordination number 6 Metal oxidation EXAMPLE: [Ni(NH 3)6]2+ Name: hexamine nickel (II) ion Coordination number : 6 Metal oxidation](https://slidetodoc.com/presentation_image_h2/2bf3fe521400395b3dba88afdbb9ee0d/image-19.jpg)
EXAMPLE: [Ni(NH 3)6]2+ Name: hexamine nickel (II) ion Coordination number : 6 Metal oxidation number : +2 Charge on complex : +2 Coordination sphere : all
![EXAMPLE Co Cl 2en2 Name dichlorobisethylenediaminecobalt III Coordination number 6 en is EXAMPLE: [Co. Cl 2(en)2]+ Name : dichlorobis(ethylenediamine)cobalt III Coordination number : 6 (en is](https://slidetodoc.com/presentation_image_h2/2bf3fe521400395b3dba88afdbb9ee0d/image-20.jpg)
EXAMPLE: [Co. Cl 2(en)2]+ Name : dichlorobis(ethylenediamine)cobalt III Coordination number : 6 (en is bidentate) Metal oxidation number : +3 Charge on complex : +1 Coordination sphere : all
![EXAMPLE Potassium amminetrichloroplatinate II Formula KPt Cl 3NH 3 Coordination number EXAMPLE: • Potassium amminetrichloroplatinate II Formula : K[Pt. Cl 3(NH 3)] Coordination number :](https://slidetodoc.com/presentation_image_h2/2bf3fe521400395b3dba88afdbb9ee0d/image-21.jpg)
EXAMPLE: • Potassium amminetrichloroplatinate II Formula : K[Pt. Cl 3(NH 3)] Coordination number : 4 Charge on complex : -1 Metal oxidation number : +2 Coordination sphere : all but K+ Counter-ions: K+