Polyatomic Ions in Compounds Polyatomic Ions Polyatomic ions








- Slides: 9

Polyatomic Ions in Compounds

Polyatomic Ions Polyatomic ions containtwo or more different atoms (polyatomic means “many atoms”). These atoms are held together bycovalent bonds, but form ions.

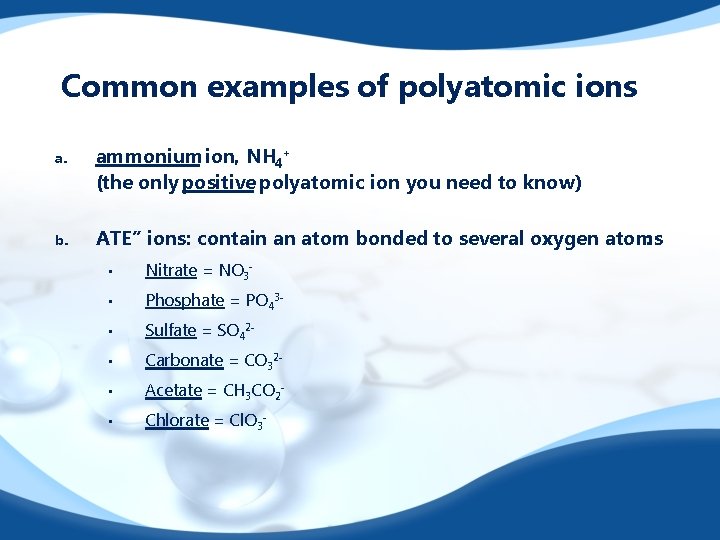
Common examples of polyatomic ions a. ammonium ion, NH 4+ (the only positive polyatomic ion you need to know) b. ATE” ions: contain an atom bonded to several oxygen atoms : • Nitrate = NO 3 - • Phosphate = PO 43 - • Sulfate = SO 42 - • Carbonate = CO 32 - • Acetate = CH 3 CO 2 - • Chlorate = Cl. O 3 -

c. “ITE” ions: remove one oxygen from the “ATE” ion and keep the same charge: • Nitrite = NO 2 - • Phosphite = PO 33 - • Sulfite = SO 32 - • Chlorite = Cl. O 2 - d. Other common complex ions: • Hydroxide = OH- • Cyanide = CN-

Ionic Compounds Containing Polyatomic Ions As you’ve already learned, ionic compounds are formed by the combination of apositive ion (cation) and a negative ion (anion). This is the same when dealing simple ions or complex ions. Complex ions are grouped together and should not be separated. In other words, don’t ever separate the sulfate ion, SO 42 - into sulfur and oxygen.

Writing Formulas Since complex ions come in groups, things can get tricky when usingsubscripts. As a result, we use brackets to separate the ion from the subscript: If we need twosulfates in a compound, we write: (SO 4)2. If we need three nitrates in a compound, we write: (NO 3)3.

And, just as before, thenet charge of the compound must be zero. +, and For a compound containing sodium ion, Na nitrate, NO 3 -, the ratio would be 1: 1 since the positive and negative charges cancel out. Therefore, the formula is. Na. NO 3 (Note: no parentheses are necessary here), and is called sodium nitrate. With polyatomic ions, do not change the name.

Example For a salt containingcalcium ion, Ca 2+, and nitrate, NO 3 -, the ratio must be 1: 2 (one calcium ion for every two nitrates). So, the formula would be. Ca(NO 3)2. How many atoms in the above formula? Ca = 1; N =2; O=6 = 9

Textbook examples: Practice Problems: