IONS AND ISOTOPES CHARACTERISTICS OF IONS Ions are



- Slides: 8

IONS AND ISOTOPES

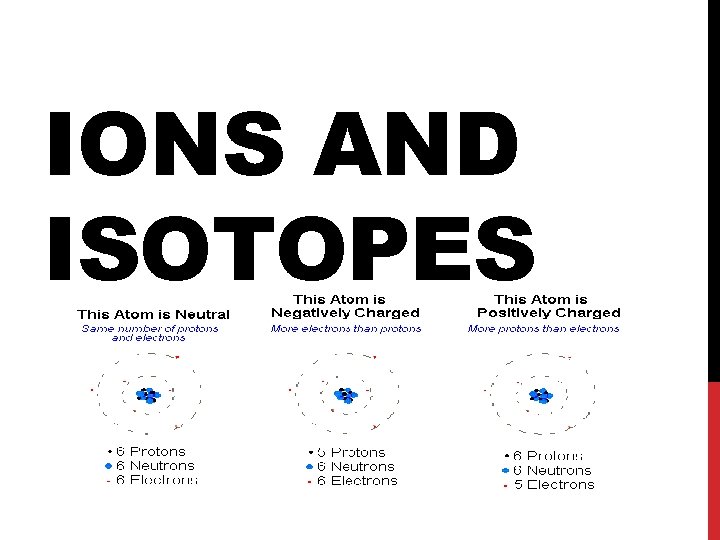
CHARACTERISTICS OF IONS Ions are particles with a +/- charge All ions begin as neutral atoms Atoms that have lost electrons are called CATIONS Atoms that have gained electrons are called ANIONS All electrons that are lost are gained are VALENCE Electrons Valence electrons are outer shell electrons that are loosely held by the atom All atoms want to have 8 valence electrons so that they can be stable Losing and gaining electrons accomplishes this during reactions

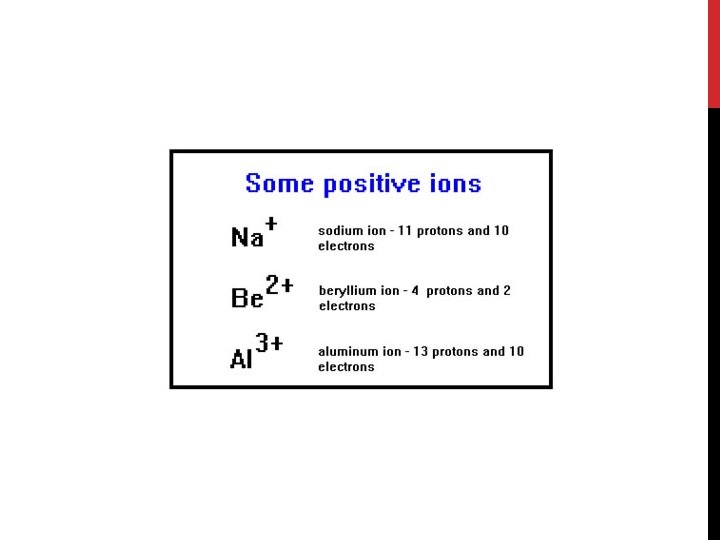
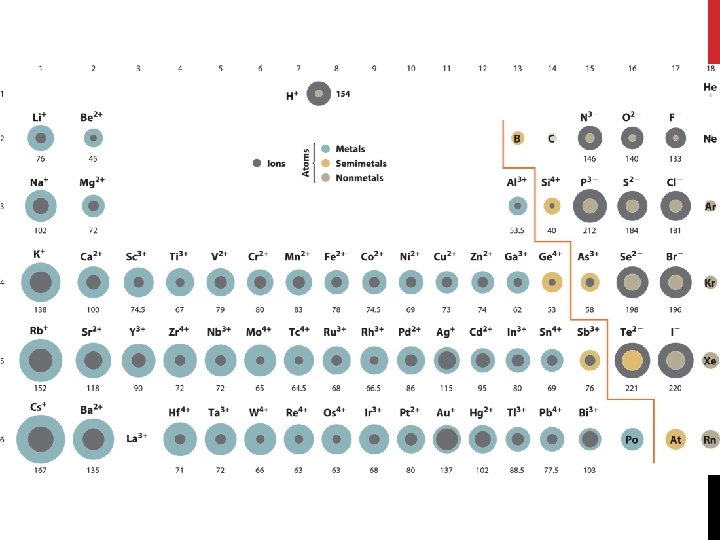
POSITIVE IONS (CATIONS) Positive ions have lost 1 or more (up to 3) valence electrons The number of electrons is less than the number of protons so the ion has a net positive charge EX: Na+1 Ion… 11 Protons and 10 Electrons 11 -10 = +1 net charge Positive ions are smaller than the atom they came from because they lose an entire energy level when they release an electron Metals ALWAYS form positive ions


NEGATIVE IONS (ANIONS) Negative ions are formed when an atom gains one or more electrons (up to 3) from another atom The number of electrons is greater in an negative ion than in the neutral atom Net charge is negative Ex: O 2 - ion 8 protons and 10 electrons 8 - 10 = -2 electrons left Negative ions are LARGER than the atom they came from because the extra electrons occupy more space NON-METALS FORM NEGATIVE IONS


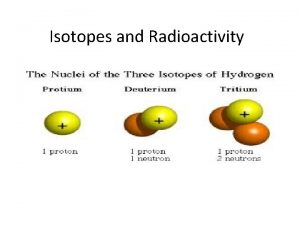
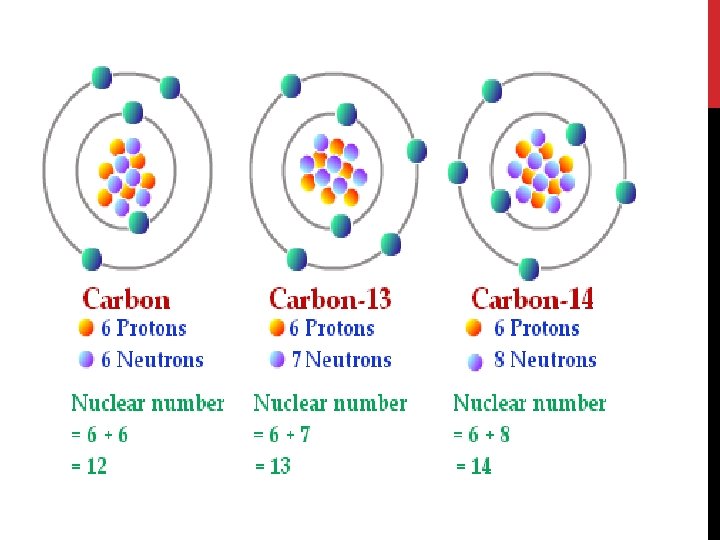
ISOTOPES Same element (same number of protons) different number of neutrons The difference in neutrons creates a sample with a different mass All elements have at least 1 isotope This is why on the periodic table we use a weighted average atomic mass to represent the mass of the element If all isotopes are not known, the mass of the element is in parenthesis (259).