Polyatomic Ions Transition Ions Polyatomic Ions Covalent structures




- Slides: 12

Polyatomic Ions Transition Ions

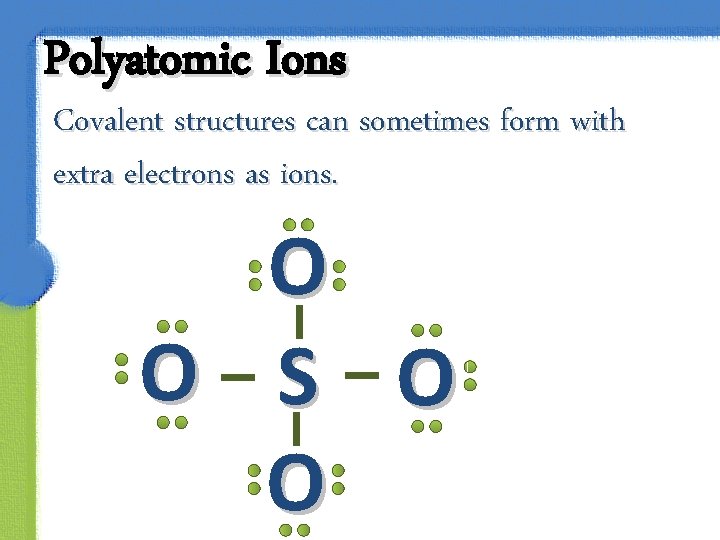
Polyatomic Ions Covalent structures can sometimes form with extra electrons as ions. O O S O O

Polyatomic Ions These covalent structures are called polyatomic ions. Many negative polyatomic ions have a central atom bound to a group of oxygen atoms which we refer to as the oxyanions.

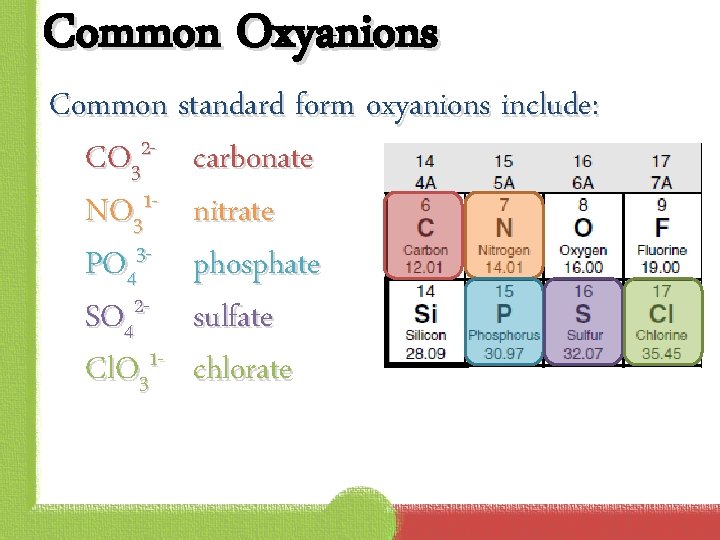
Common Oxyanions Common standard form oxyanions include: CO 32 - carbonate 1 NO 3 nitrate 3 PO 4 phosphate SO 42 - sulfate Cl. O 31 - chlorate

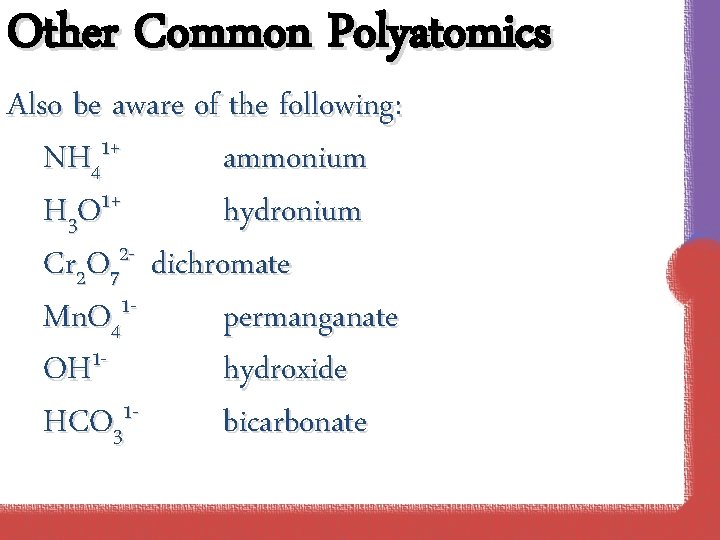
Other Common Polyatomics Also be aware of the following: NH 41+ ammonium 1+ H 3 O hydronium 2 Cr 2 O 7 dichromate Mn. O 41 permanganate OH 1 hydroxide HCO 31 bicarbonate

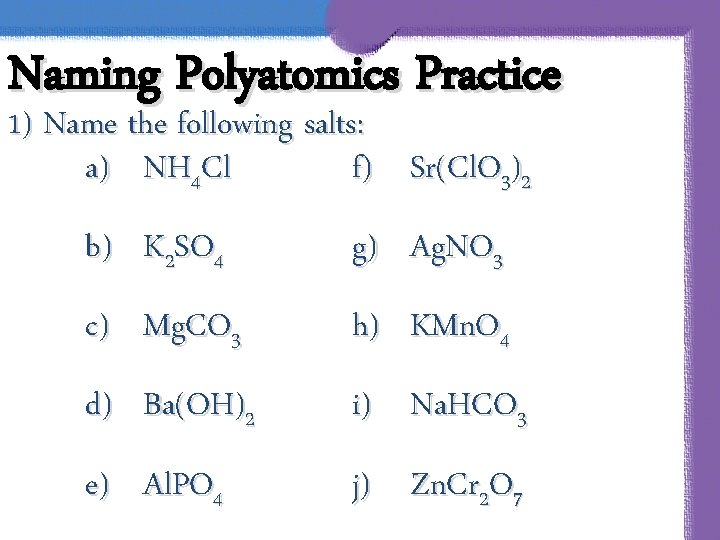
Naming Polyatomics Practice 1) Name the following salts: a) NH 4 Cl f) Sr(Cl. O 3)2 b) K 2 SO 4 g) Ag. NO 3 c) Mg. CO 3 h) KMn. O 4 d) Ba(OH)2 i) Na. HCO 3 e) Al. PO 4 j) Zn. Cr 2 O 7

Chemical Formula Practice 2) Write chemical formulas for the salts below: a) lithium sulfate f) potassium dichromate b) barium chlorate g) cesium bicarbonate c) h) sodium permanganate aluminum hydroxide d) strontium carbonate i) magnesium phosphate e) j) ammonium hydroxide sodium nitrate

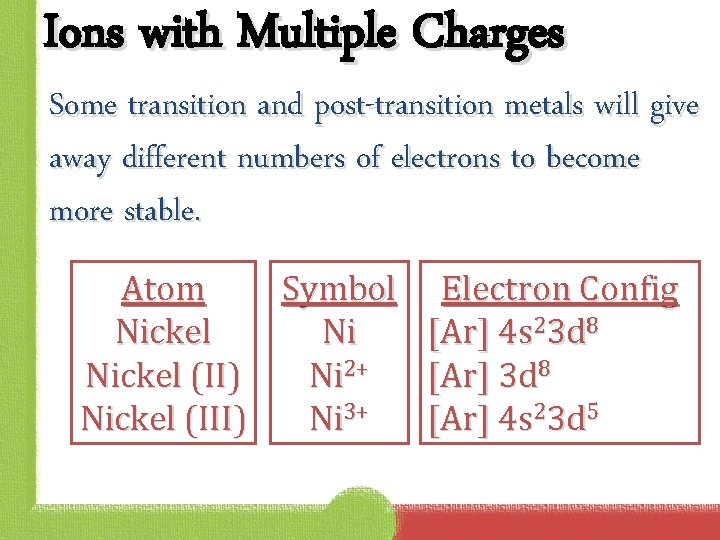
Ions with Multiple Charges Some transition and post-transition metals will give away different numbers of electrons to become more stable. Atom Symbol Electron Config Nickel Ni [Ar] 4 s 23 d 8 Nickel (II) Ni 2+ [Ar] 3 d 8 Nickel (III) Ni 3+ [Ar] 4 s 23 d 5

Common Transition Ions Element Ion Stock Name Classical Name chromium Cr 2+ chromium(II) chromous Cr 3+ chromium(III) chromic cobalt Co 2+ Co 3+ cobalt(II) cobalt(III) cobaltous cobaltic copper Cu 1+ Cu 2+ copper(I) copper(II) cuprous cupric iron Fe 2+ iron(II) ferrous Fe 3+ iron(III) ferric Pb 2+ lead(II) plumbous Pb 4+ Mn 2+ Mn 3+ Mn 4+ lead(IV) manganese(III) manganese(IV) plumbic manganous manganic Hg 22+ mercury(I) mercurous Hg 2+ mercury(II) mercuric nickel Ni 2+ Ni 3+ nickel(II) nickel(III) nickelous nickelic tin Sn 2+ tin(II) stannous Sn 4+ tin(IV) stannic lead manganese mercury

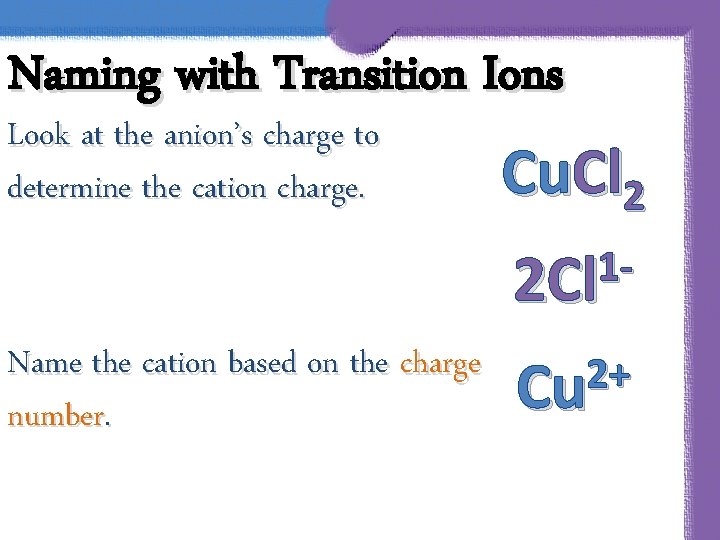
Naming with Transition Ions Look at the anion’s charge to determine the cation charge. Cu. Cl 2 12 Cl Name the cation based on the charge number. 2+ Cu

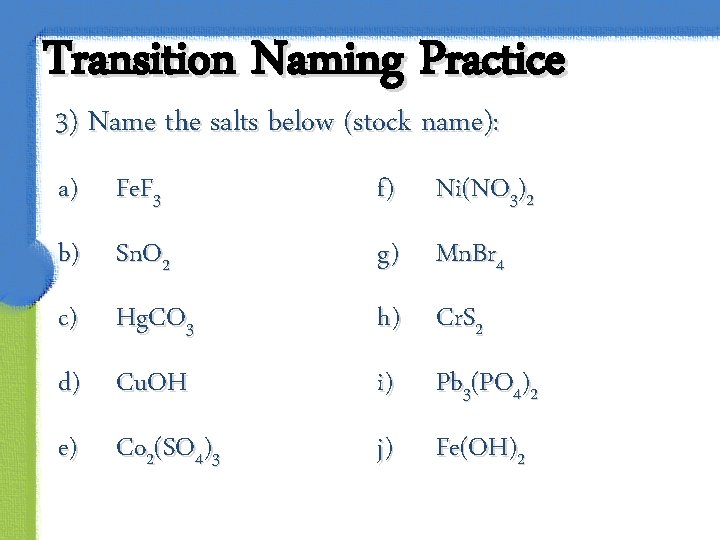
Transition Naming Practice 3) Name the salts below (stock name): a) Fe. F 3 f) Ni(NO 3)2 b) Sn. O 2 g) Mn. Br 4 c) h) Cr. S 2 Hg. CO 3 d) Cu. OH i) Pb 3(PO 4)2 e) j) Fe(OH)2 Co 2(SO 4)3

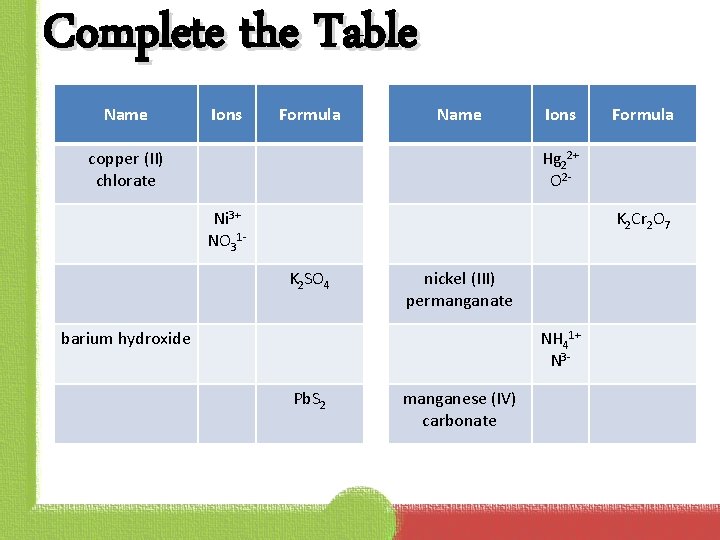
Complete the Table Name Ions Formula Name copper (II) chlorate Ions Formula Hg 22+ O 2 Ni 3+ NO 31 - K 2 Cr 2 O 7 K 2 SO 4 nickel (III) permanganate barium hydroxide NH 41+ N 3 Pb. S 2 manganese (IV) carbonate