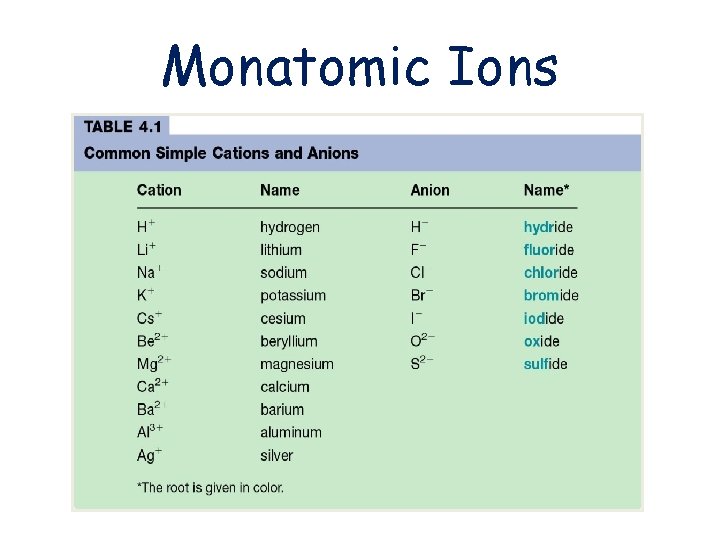
IONIC COMPOUNDS Naming and Formula Writing Monatomic Ions













- Slides: 27

IONIC COMPOUNDS Naming and Formula Writing

Monatomic Ions

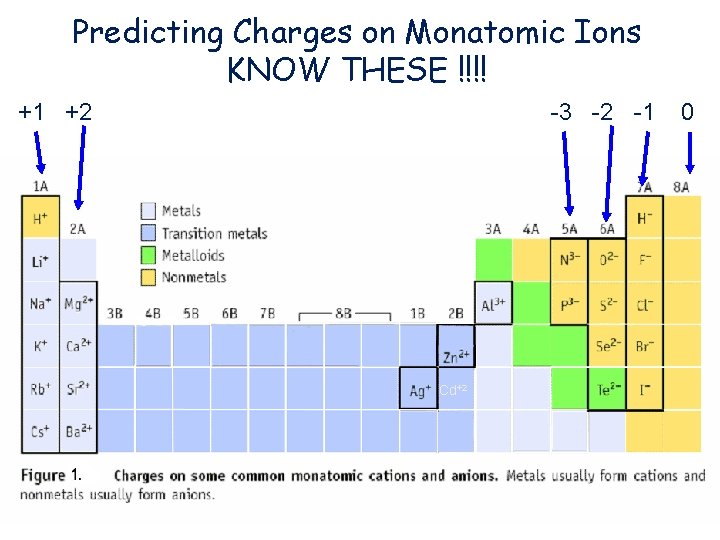
Predicting Charges on Monatomic Ions KNOW THESE !!!! +1 +2 -3 -2 -1 Cd+2 0

Naming Positive Ions • Before you name an Ion you have to know the charge • Group 1 = always +1 • Group 2 = always +2 • Aluminum = always +3 • Zinc and Cadmium = always +2 • Silver = always +1

Naming Positive Ions • With group 1, 2, Al, Zn, Cd, and Ag you just give the name of the atom – Na+ is Sodium – Mg+2 is Magnesium – Ag+ is Silver – Al+3 is Aluminum

Naming Negative Ions • All negative ions have their endings changed to –ide • Oxygen becomes oxide • Fluorine becomes Fluoride • Nitrogen becomes Nitride • Chlorine becomes Chloride

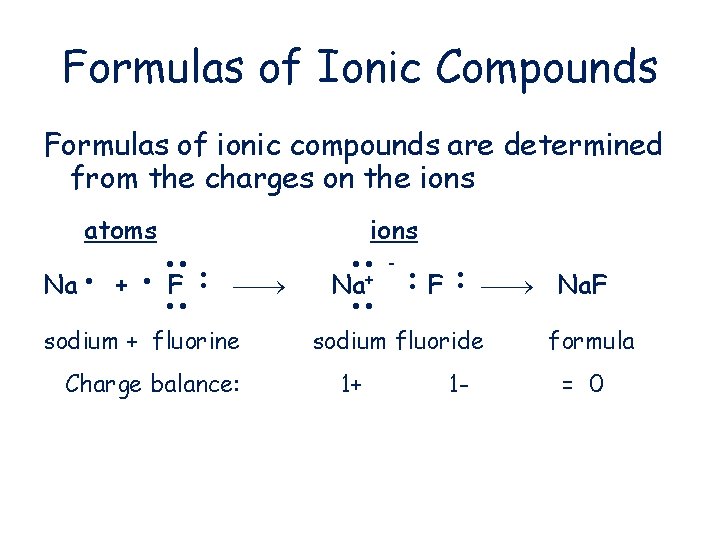
Formulas of Ionic Compounds Formulas of ionic compounds are determined from the charges on the ions atoms F sodium + fluorine Charge balance: F + Na+ Na ions Na. F sodium fluoride 1+ 1 - formula = 0

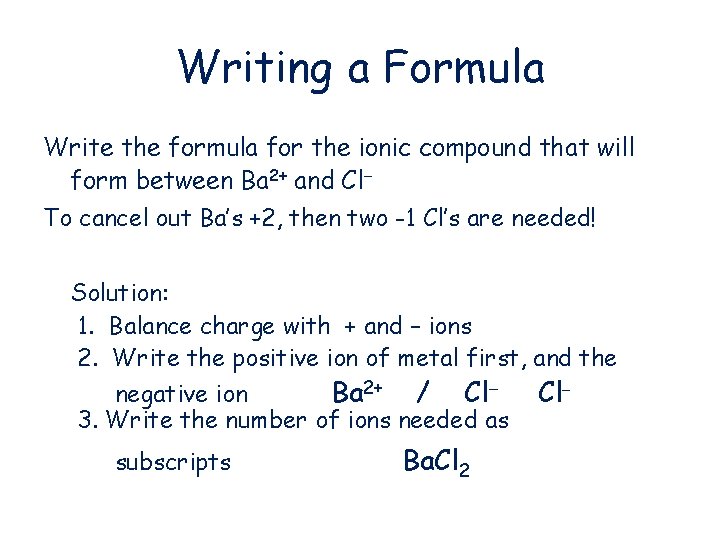
Writing a Formula Write the formula for the ionic compound that will form between Ba 2+ and Cl To cancel out Ba’s +2, then two -1 Cl’s are needed! Solution: 1. Balance charge with + and – ions 2. Write the positive ion of metal first, and the negative ion Ba 2+ / Cl 3. Write the number of ions needed as subscripts Ba. Cl 2 Cl

Balancing a formula by math • Every ionic compound should have a formula that has a charge that equals zero • Barium Fluoride – Ba+2 F– How many F ’s are needed to balance Ba+2 ? – Two – So, the Formula is Ba. F 2

Another way – Drop, Swap, Reduce What is the formula for Aluminum Oxide? Al is always +3 is r C “ Oxide is always -2 d e l al c o ! Al+3, O-2 Als ross” —C DROP: Al 3 O 2 SWAP: Al 2 O 3 REDUCE: 2 and 3 are lowest integers, so leave alone • The Final Formula is Al 2 O 3 • •

Another example Magnesium Oxide Mg is +2 Oxide is -2 Mg+2, O-2 DROP: Mg 2 O 2 SWAP: Mg 2 O 2 REDUCE: Mg. O, 2 and 2 divide each other out • The Final Formula is Mg. O • •

Learning Check Which is the correct formula for the compounds containing the following ions: 1. Na+, S 2 a) Na. S 2. Al 3+, Cla) Al. Cl 3 3. Mg 2+, N 3 a) Mg. N b) Na 2 S c) Na. S 2 b) Al. Cl c) Al 3 Cl b) Mg 2 N 3 c) Mg 3 N 2

Solution 1. Na+, S 2 b) Na 2 S 2. Al 3+, Cla) Al. Cl 3 3. Mg 2+, N 3 c) Mg 3 N 2

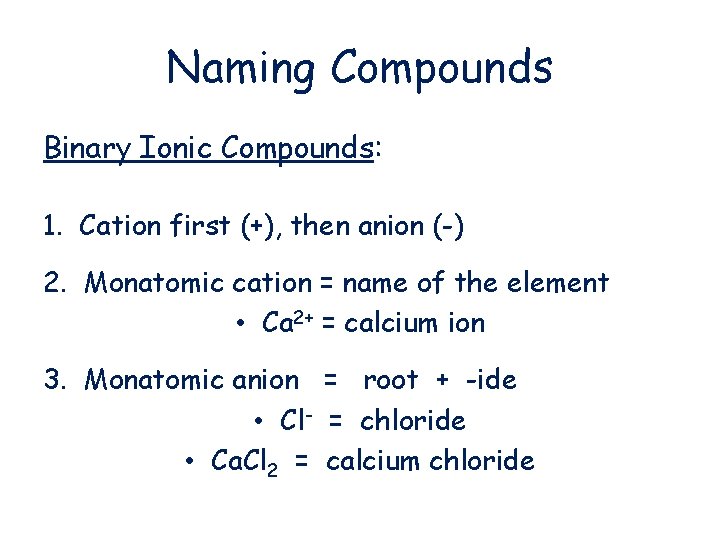
Naming Compounds Binary Ionic Compounds: 1. Cation first (+), then anion (-) 2. Monatomic cation = name of the element • Ca 2+ = calcium ion 3. Monatomic anion = root + -ide • Cl- = chloride • Ca. Cl 2 = calcium chloride

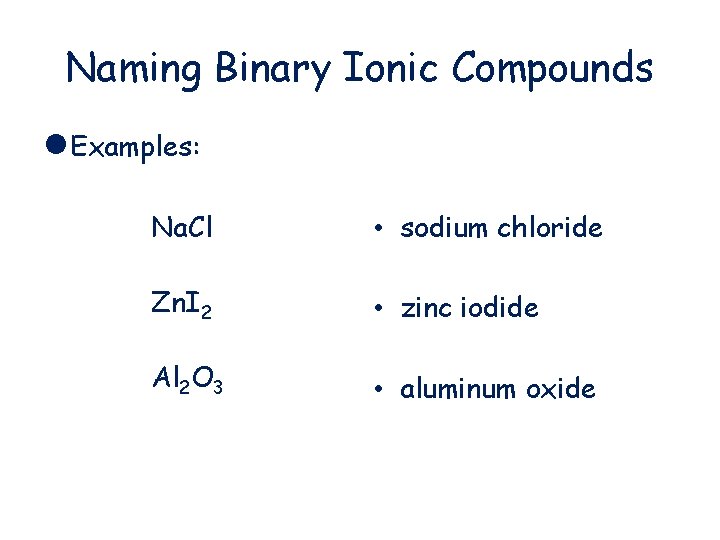
Naming Binary Ionic Compounds l Examples: Na. Cl • sodium chloride Zn. I 2 • zinc iodide Al 2 O 3 • aluminum oxide

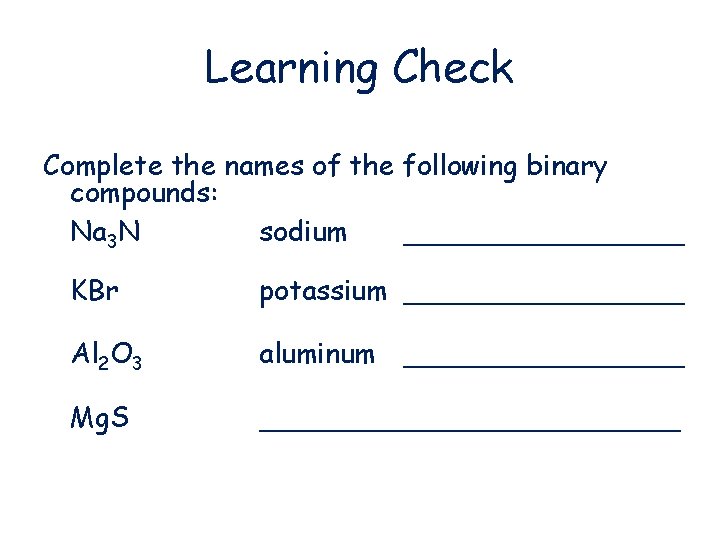
Learning Check Complete the names of the following binary compounds: Na 3 N sodium ________ KBr potassium ________ Al 2 O 3 aluminum ________ Mg. S ____________

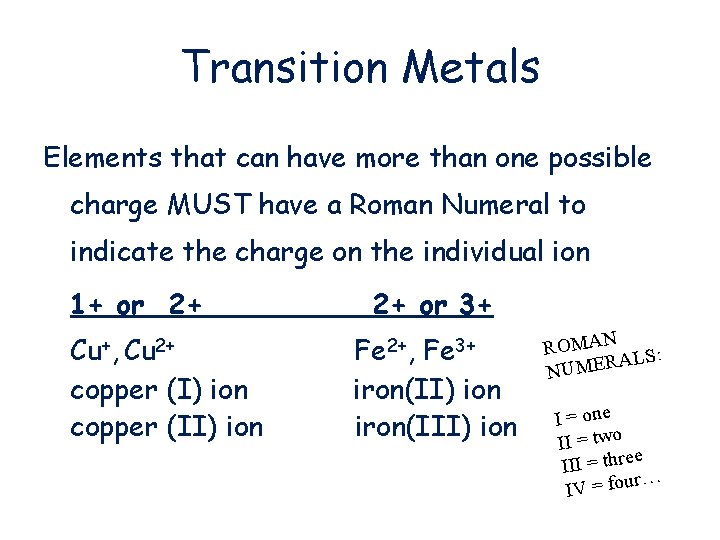
Transition Metals Elements that can have more than one possible charge MUST have a Roman Numeral to indicate the charge on the individual ion 1+ or 2+ Cu+, Cu 2+ copper (I) ion copper (II) ion 2+ or 3+ Fe 2+, Fe 3+ iron(II) ion iron(III) ion ROMAN S: AL NUMER I = one II = two e III = thre r… IV = fou

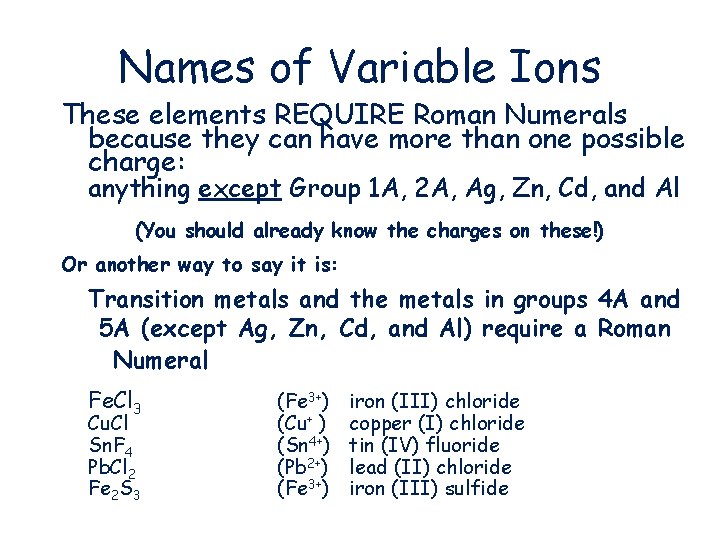
Names of Variable Ions These elements REQUIRE Roman Numerals because they can have more than one possible charge: anything except Group 1 A, 2 A, Ag, Zn, Cd, and Al (You should already know the charges on these!) Or another way to say it is: Transition metals and the metals in groups 4 A and 5 A (except Ag, Zn, Cd, and Al) require a Roman Numeral Fe. Cl 3 Cu. Cl Sn. F 4 Pb. Cl 2 Fe 2 S 3 (Fe 3+) (Cu+ ) (Sn 4+) (Pb 2+) (Fe 3+) iron (III) chloride copper (I) chloride tin (IV) fluoride lead (II) chloride iron (III) sulfide

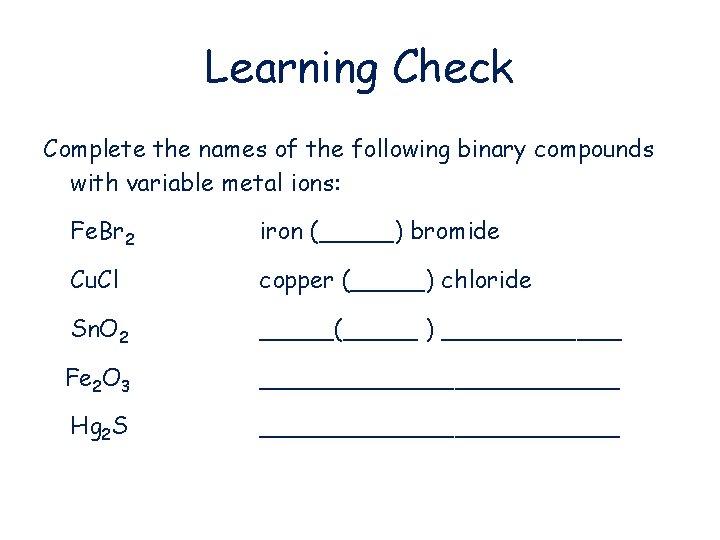
Learning Check Complete the names of the following binary compounds with variable metal ions: Fe. Br 2 iron (_____) bromide Cu. Cl copper (_____) chloride Sn. O 2 _____(_____ ) ______ Fe 2 O 3 ____________ Hg 2 S ____________

Polyatomic Ions • Some ions are composed of more then one atom • These are called polyatomic ions – Poly = more

Formulas and names • Nitrate = NO 3 • Sulfate = SO 4 -2 • Silver nitrate – Ag+ NO 3– Ag. NO 3 • Copper (I) Sulfate – Cu+ SO 4 -2 – Cu 2 SO 4

More Polyatomics • Lead (IV) Phosphate – Pb+4 PO 4 -3 – Pb 3(PO 4)4 • Notice: When more then one Polyatomic is present you surround it with () • Notice: The subscripts on Polyatomic ions are NEVER changed

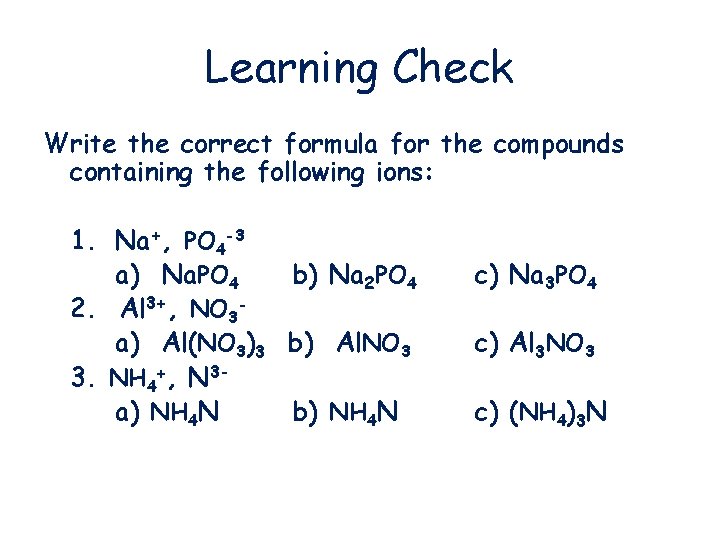
Learning Check Write the correct formula for the compounds containing the following ions: 1. Na+, PO 4 -3 a) Na. PO 4 b) Na 2 PO 4 2. Al 3+, NO 3 a) Al(NO 3)3 b) Al. NO 3 3. NH 4+, N 3 a) NH 4 N b) NH 4 N c) Na 3 PO 4 c) Al 3 NO 3 c) (NH 4)3 N

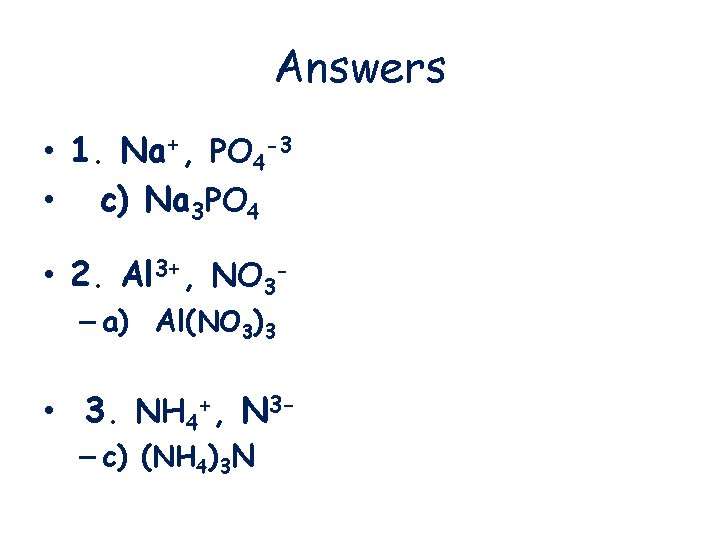
Answers • 1. Na+, PO 4 -3 • c) Na 3 PO 4 • 2. Al 3+, NO 3– a) Al(NO 3)3 • 3. NH 4+, N 3– c) (NH 4)3 N

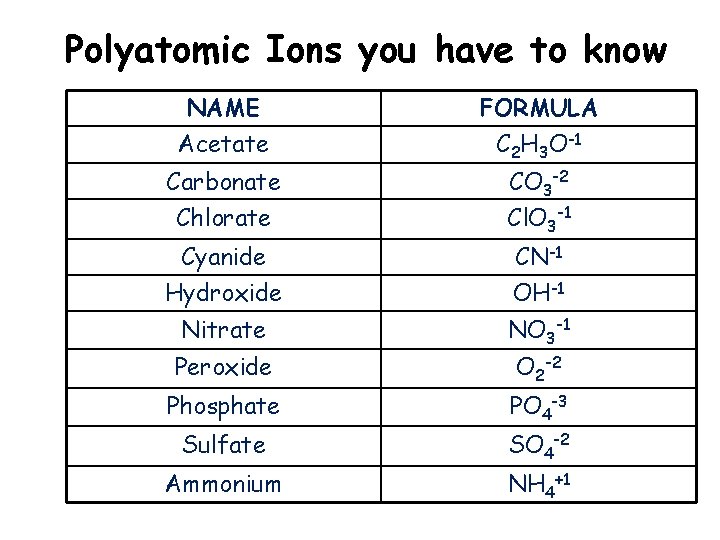
Polyatomic Ions you have to know NAME Acetate FORMULA C 2 H 3 O-1 Carbonate Chlorate CO 3 -2 Cl. O 3 -1 Cyanide CN-1 Hydroxide OH-1 Nitrate NO 3 -1 Peroxide O 2 -2 Phosphate PO 4 -3 Sulfate SO 4 -2 Ammonium NH 4+1

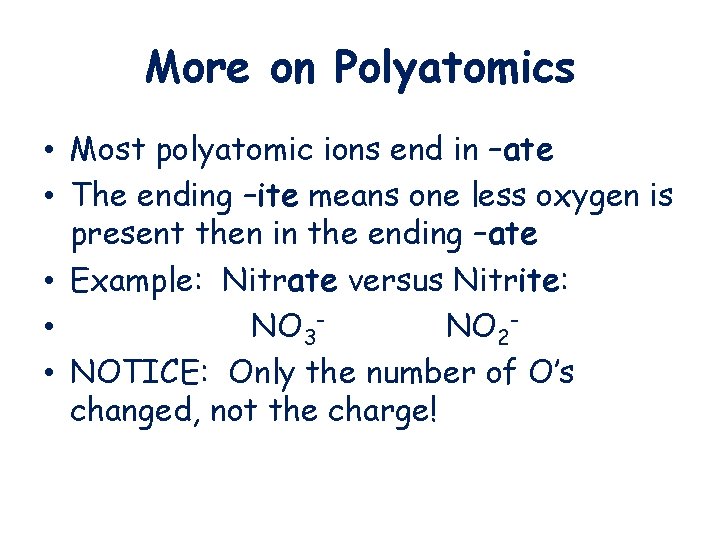
More on Polyatomics • Most polyatomic ions end in –ate • The ending –ite means one less oxygen is present then in the ending –ate • Example: Nitrate versus Nitrite: • NO 3 NO 2 • NOTICE: Only the number of O’s changed, not the charge!

Properties of Ionic Compounds • Ionic compounds are: – also known as salts – They are usually hard and brittle – Conduct electricity when molten or dissolved – Have very high melting and boiling points – Most are soluble in water – Normally composed of at least one metal and one nonmetal