POLYATOMIC IONS Polyatomic Ions A polyatomic ion is
















- Slides: 22


POLYATOMIC IONS

Polyatomic Ions • A polyatomic ion is a group of atoms together in groups (like a package of atoms) and have a charge like an ion. • Most polyatomic ions are negative and they behave like a single unit in a compound. • The most common positive polyatomic ion is: NH 4+ • It behaves like a METALS in a compound.

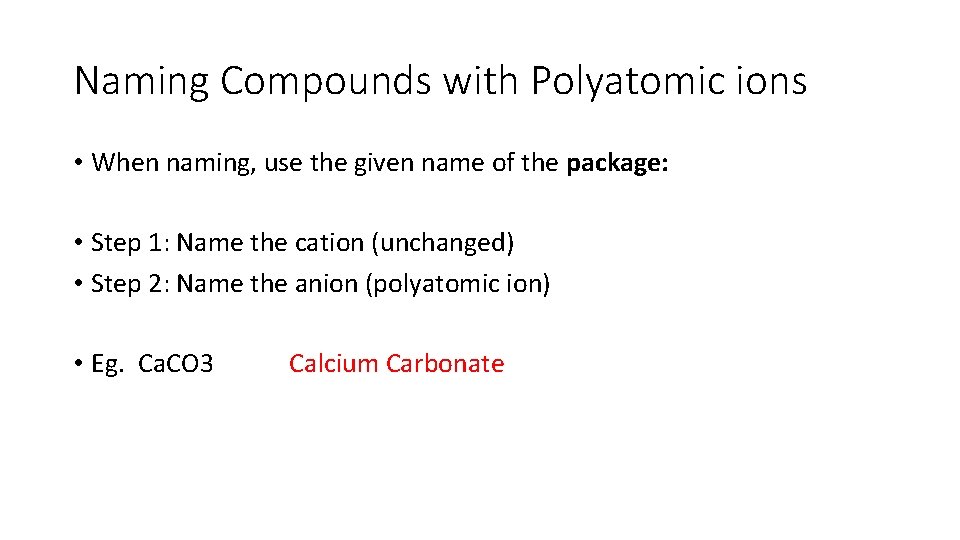
Naming Compounds with Polyatomic ions • When naming, use the given name of the package: • Step 1: Name the cation (unchanged) • Step 2: Name the anion (polyatomic ion) • Eg. Ca. CO 3

Naming Compounds with Polyatomic ions • When naming, use the given name of the package: • Step 1: Name the cation (unchanged) • Step 2: Name the anion (polyatomic ion) • Eg. Ca. CO 3 Calcium Carbonate

Try these • Na. NO 3 • K 2 Cr. O 4 • KOH • Li. NO 2

Try these • Na. NO 3 • K 2 Cr. O 4 • KOH • Li. NO 2 Sodium nitrate Potassium chromate Potassium hydroxide Lithium nitrite

POLYATOMIC IONS • Use brackets around the whole polyatomic group if more than one is needed, and the subscript OUTSIDE of the bracket. • The subscript refers to everything inside the brackets. • Example: Al 2(CO 3)3

POLYATOMIC IONS • Example: Al 2(CO 3)3 • How many Oxygen in this formula?

POLYATOMIC IONS • Example: Al 2(CO 3)3 • How many Oxygen in this formula? • 3 x 3 = 9 Oxygen • How many Aluminum?

POLYATOMIC IONS • Example: Al 2(CO 3)3 • How many Oxygen in this formula? • 3 x 3 = 9 Oxygen • How many Aluminum? TWO • THREE carbon

More Practice Mg. SO 4 Ca(NO 3)2 Li 2 SO 3 Au 2 CO 3

Practice Mg. SO 4 Magnesium Sulfate Ca(NO 3)2 Calcium Nitrate Li 2 SO 3 Lithium Sulfite Au 2 CO 3 Gold Carbonate

One positive Polyatomic Ion • NH 4 + Ammonium ion • NH 4 Cl • Step 1: Name the cation first in this case, keep the name of the polyatomic ion. • Step 2: Name the anion (change the ending to –ide).

One positive Polyatomic Ion NH 4 Cl • Ammonium Chloride

Writing Formulas using the Cross Over Method

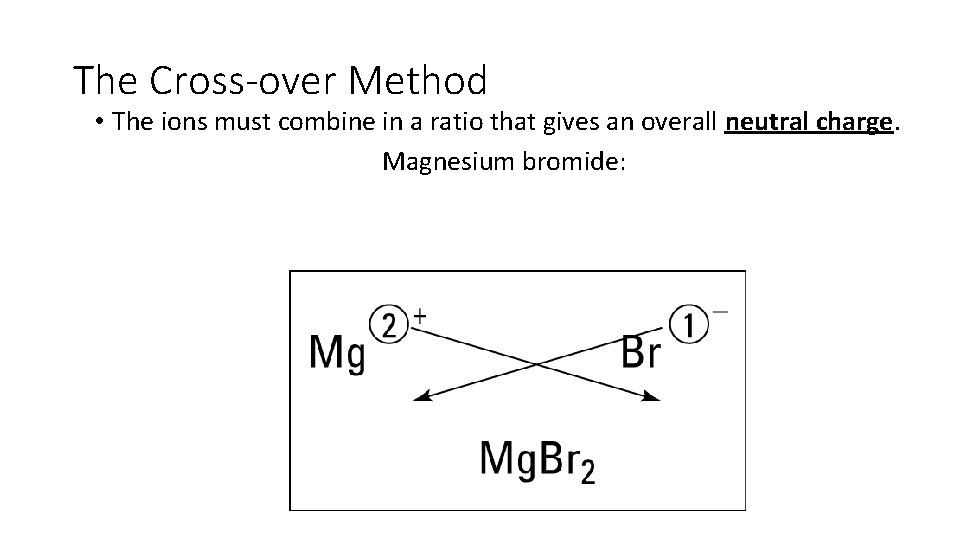
The Cross-over Method • The ions must combine in a ratio that gives an overall neutral charge. Magnesium bromide:

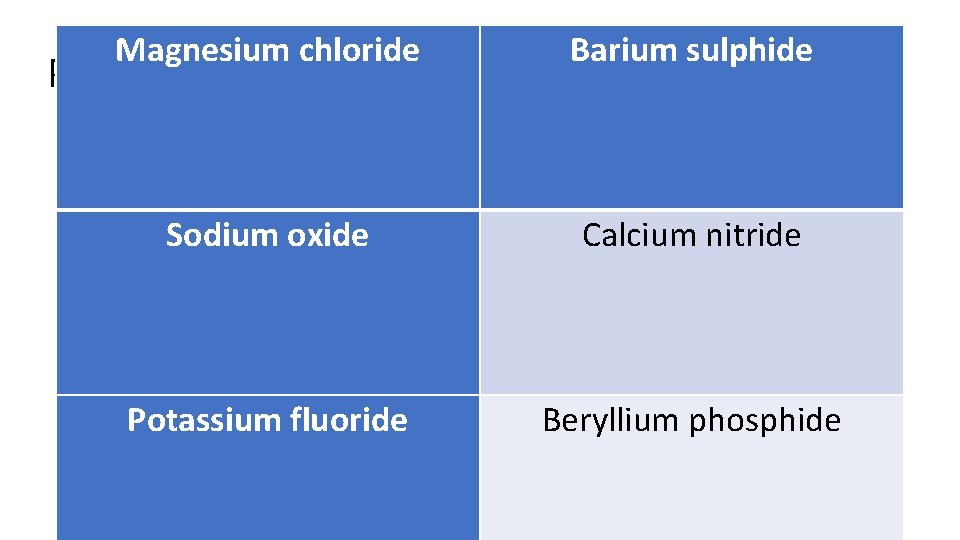
Magnesium chloride Barium sulphide Sodium oxide Calcium nitride Potassium fluoride Beryllium phosphide Practice

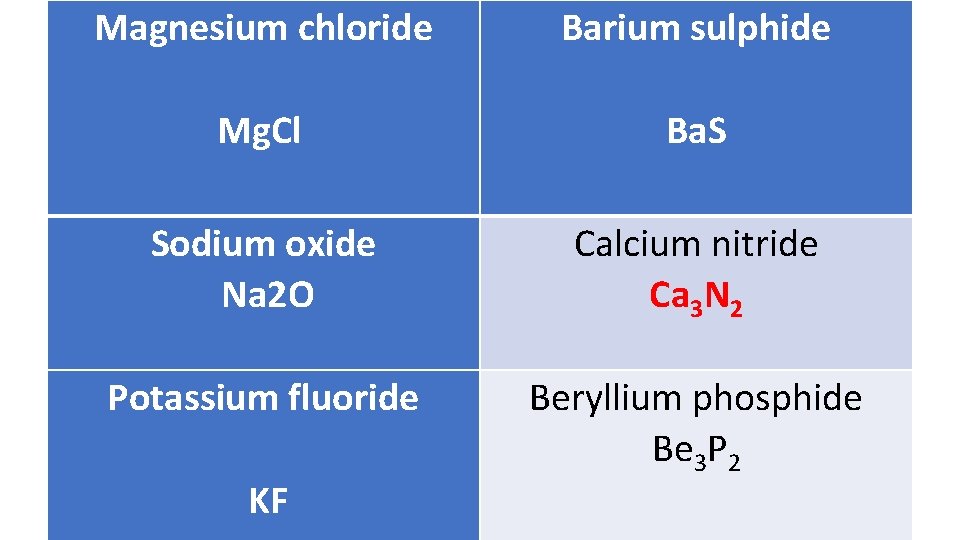
Magnesium chloride Barium sulphide Mg. Cl Ba. S Sodium oxide Na 2 O Calcium nitride Ca 3 N 2 Potassium fluoride Beryllium phosphide Be 3 P 2 Practice KF

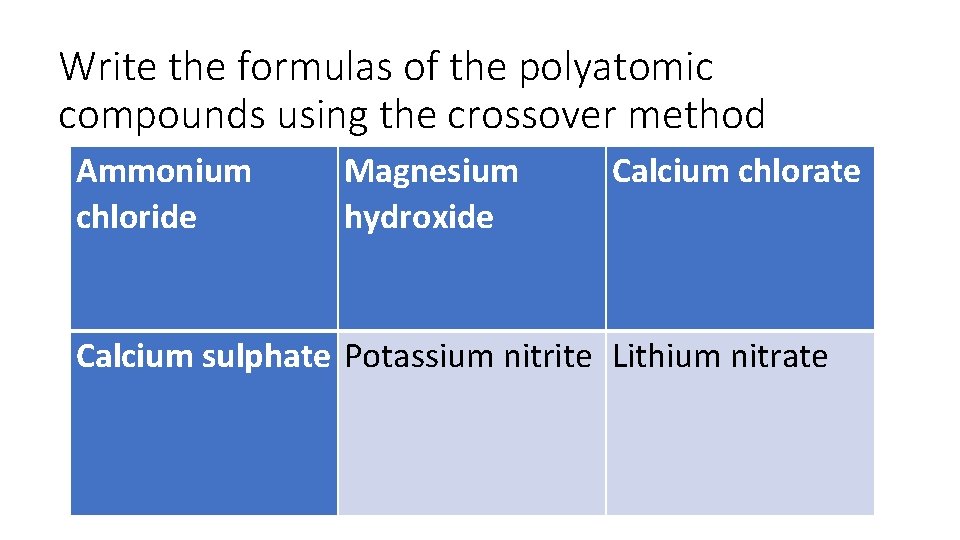
Write the formulas of the polyatomic compounds using the crossover method Ammonium chloride Magnesium hydroxide Calcium chlorate Calcium sulphate Potassium nitrite Lithium nitrate

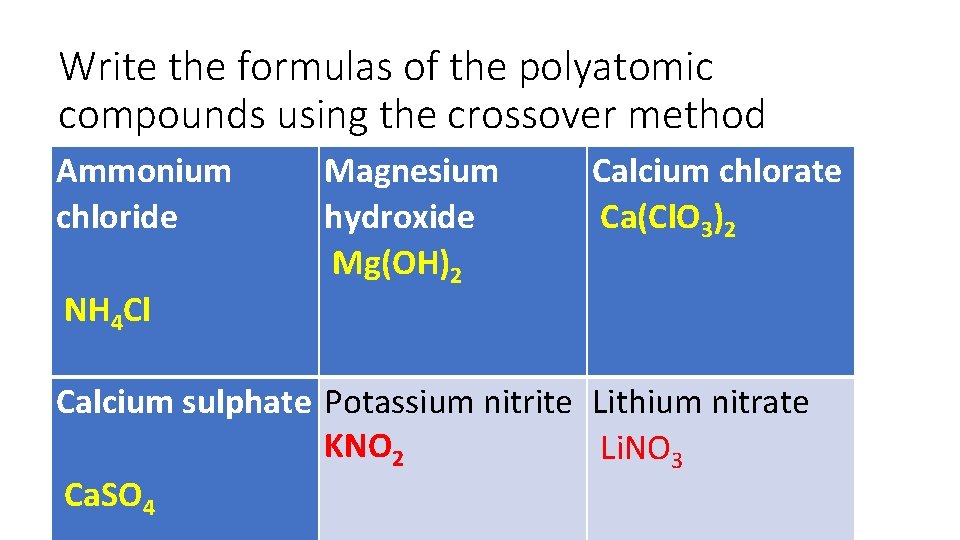
Write the formulas of the polyatomic compounds using the crossover method Ammonium chloride NH 4 Cl Magnesium hydroxide Mg(OH)2 Calcium chlorate Ca(Cl. O 3)2 Calcium sulphate Potassium nitrite Lithium nitrate KNO 2 Li. NO 3 Ca. SO 4

Homework • Read text p 238 -241 • Answer CYU p 244 #1, 6, 10 a, b, d, e, 11 bcdf, 12 abcd • WB page 46