Chemistry Jan 22 2018 Do Now Polyatomic quiz

- Slides: 9

Chemistry – Jan 22, 2018 Do Now – Polyatomic quiz trial 4 Objective – Agenda Polyatomic ion quiz Compound Identification All Compounds Identification Covalent Nomenclature (1) Covalent compound nomenclature Acid Nomenclature (3) Acid Nomenclature Mixed Practice Assignment: All Compounds Nomenclature Worksheet

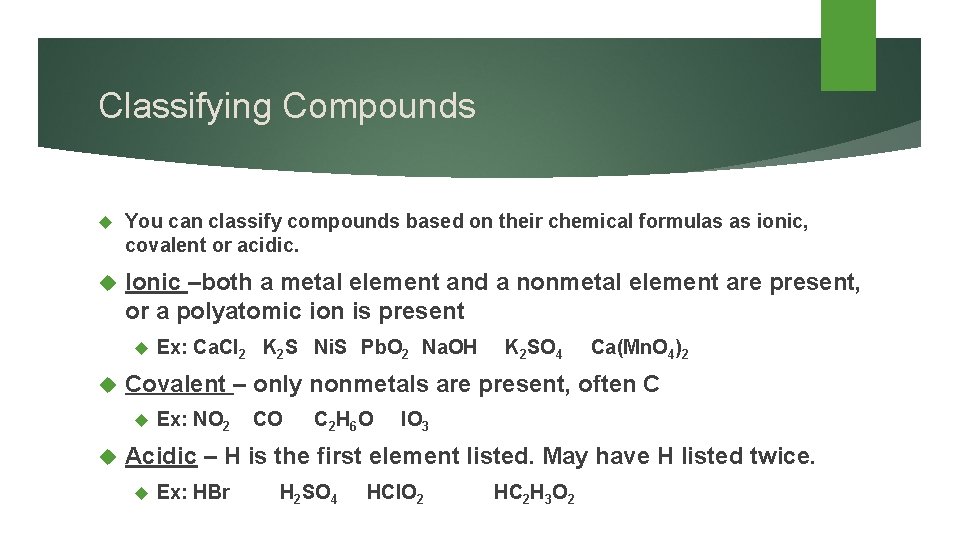
Classifying Compounds You can classify compounds based on their chemical formulas as ionic, covalent or acidic. Ionic –both a metal element and a nonmetal element are present, or a polyatomic ion is present Covalent – only nonmetals are present, often C Ex: Ca. Cl 2 K 2 S Ni. S Pb. O 2 Na. OH K 2 SO 4 Ca(Mn. O 4)2 Ex: NO 2 CO C 2 H 6 O IO 3 Acidic – H is the first element listed. May have H listed twice. Ex: HBr H 2 SO 4 HCl. O 2 HC 2 H 3 O 2

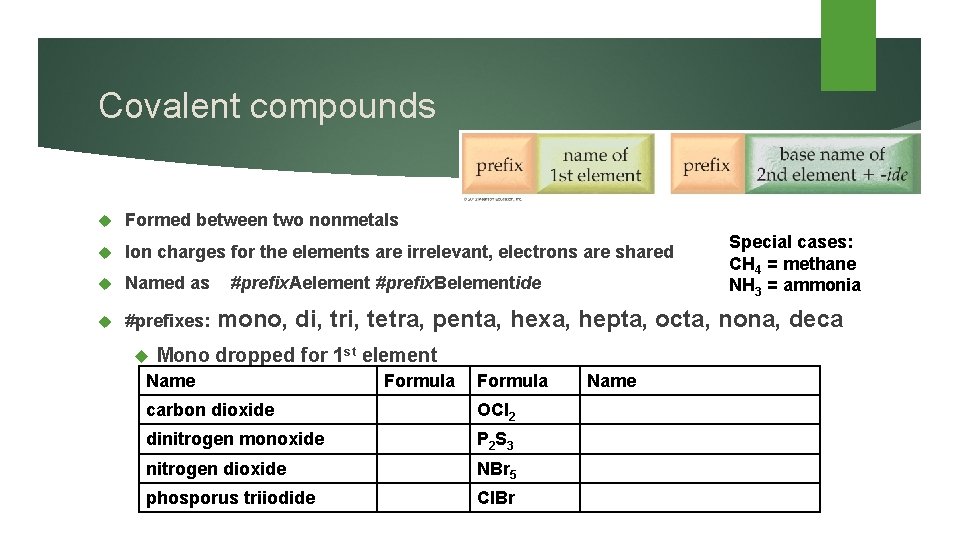
Covalent compounds Formed between two nonmetals Ion charges for the elements are irrelevant, electrons are shared Named as #prefix. Aelement #prefix. Belementide #prefixes: mono, di, tri, tetra, penta, hexa, hepta, octa, nona, deca Mono dropped for 1 st element Name Formula carbon dioxide OCl 2 dinitrogen monoxide P 2 S 3 nitrogen dioxide NBr 5 phosporus triiodide Cl. Br Name Special cases: CH 4 = methane NH 3 = ammonia

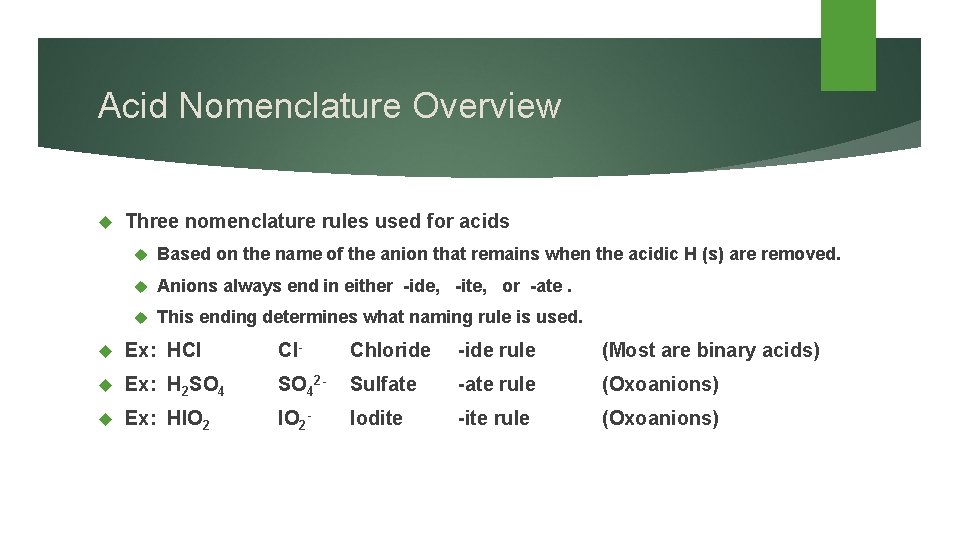
Acid Nomenclature Overview Three nomenclature rules used for acids Based on the name of the anion that remains when the acidic H (s) are removed. Anions always end in either -ide, -ite, or -ate. This ending determines what naming rule is used. Ex: HCl Cl- Ex: H 2 SO 4 Ex: HIO 2 Chloride -ide rule (Most are binary acids) SO 42 - Sulfate -ate rule (Oxoanions) IO 2 - -ite rule (Oxoanions) Iodite

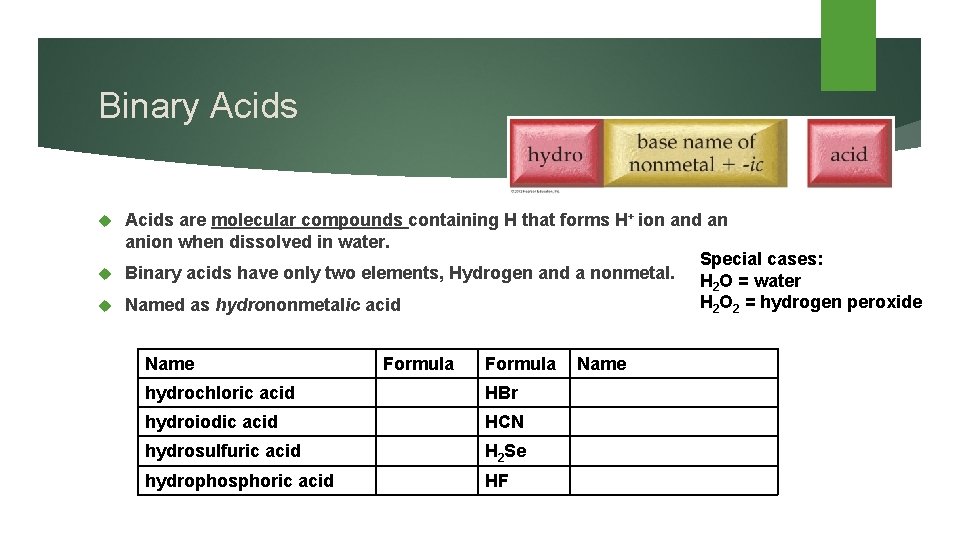
Binary Acids are molecular compounds containing H that forms H+ ion and an anion when dissolved in water. Special cases: Binary acids have only two elements, Hydrogen and a nonmetal. H 2 O = water H 2 O 2 = hydrogen peroxide Named as hydrononmetalic acid Name Formula hydrochloric acid HBr hydroiodic acid HCN hydrosulfuric acid H 2 Se hydrophosphoric acid HF Name

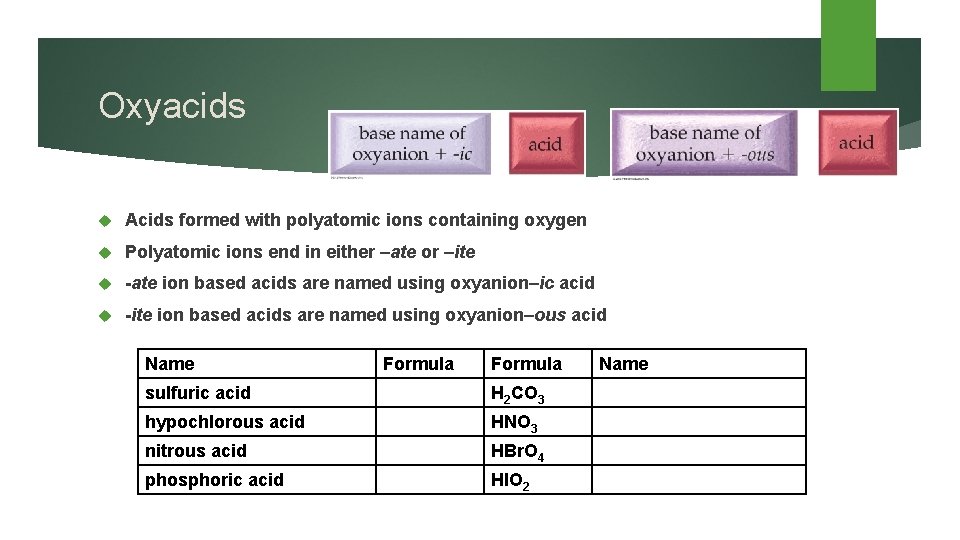
Oxyacids Acids formed with polyatomic ions containing oxygen Polyatomic ions end in either –ate or –ite -ate ion based acids are named using oxyanion–ic acid -ite ion based acids are named using oxyanion–ous acid Name Formula sulfuric acid H 2 CO 3 hypochlorous acid HNO 3 nitrous acid HBr. O 4 phosphoric acid HIO 2 Name

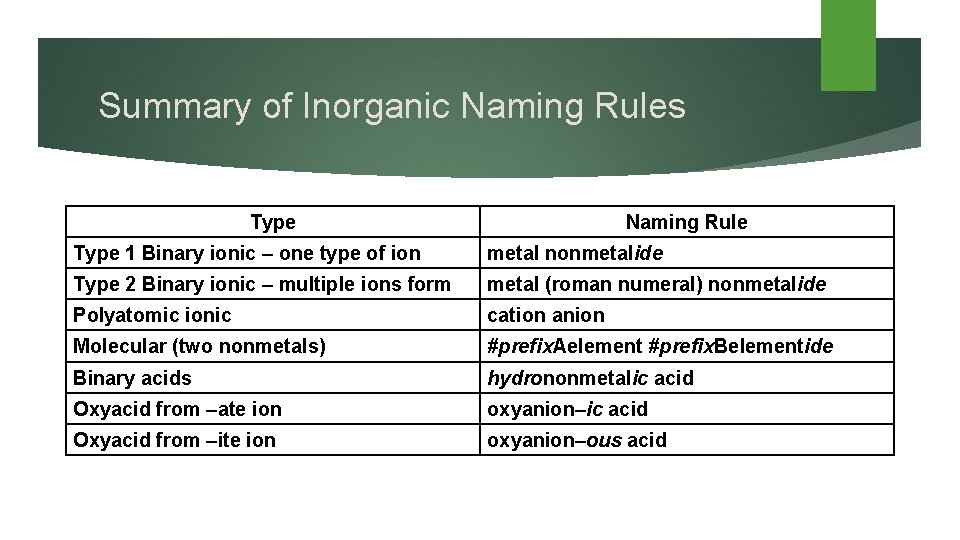
Summary of Inorganic Naming Rules Type Naming Rule Type 1 Binary ionic – one type of ion metal nonmetalide Type 2 Binary ionic – multiple ions form metal (roman numeral) nonmetalide Polyatomic ionic cation anion Molecular (two nonmetals) #prefix. Aelement #prefix. Belementide Binary acids hydrononmetalic acid Oxyacid from –ate ion oxyanion–ic acid Oxyacid from –ite ion oxyanion–ous acid

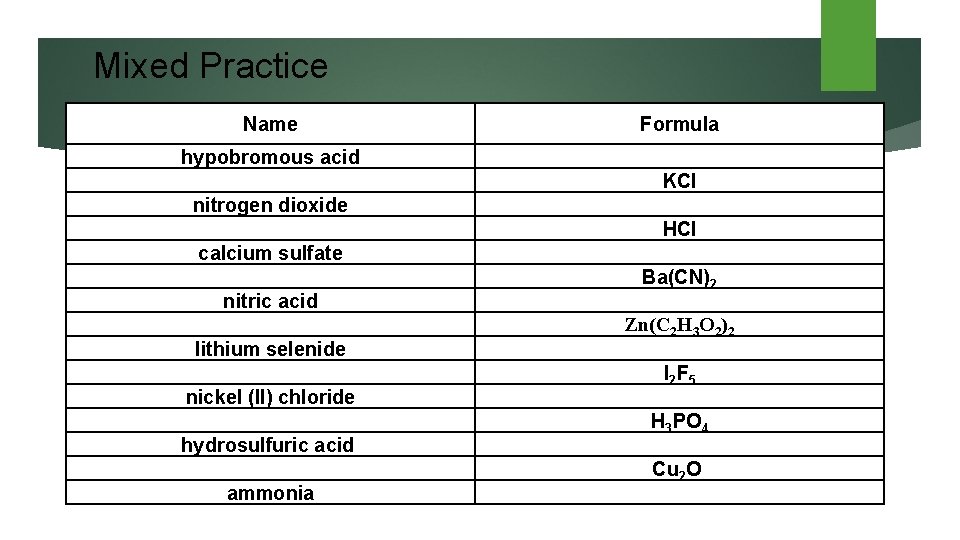
Mixed Practice Name Formula hypobromous acid nitrogen dioxide calcium sulfate nitric acid KCl HCl Ba(CN)2 Zn(C 2 H 3 O 2)2 lithium selenide nickel (II) chloride hydrosulfuric acid ammonia I 2 F 5 H 3 PO 4 Cu 2 O

Exit Slip - Homework Exit Slip: Name: What’s the formula for hypobromous acid What’s Due? (Pending assignments to complete. ) P 2 O 5 All Compounds Nomenclature Worksheet What’s Next? (How to prepare for the next day) Read Holt p 159 -175 Mixed Nomenclature quiz on Monday (Just like the mixed Practice).