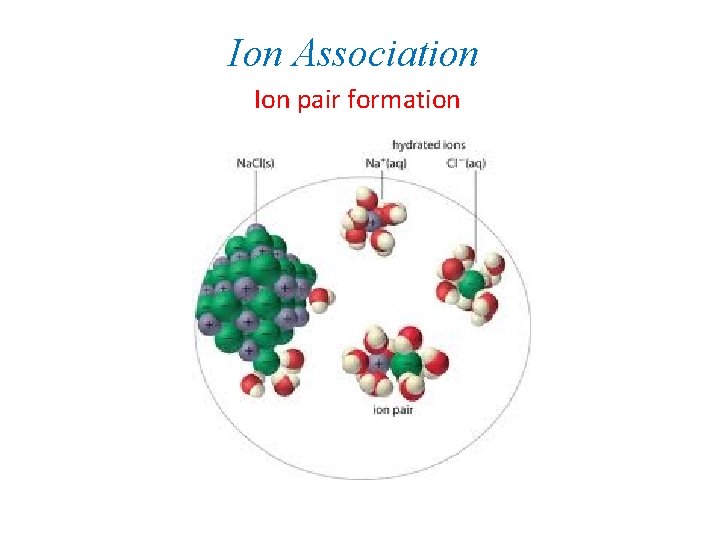
Ion Association Ion pair formation Ion Association Ion


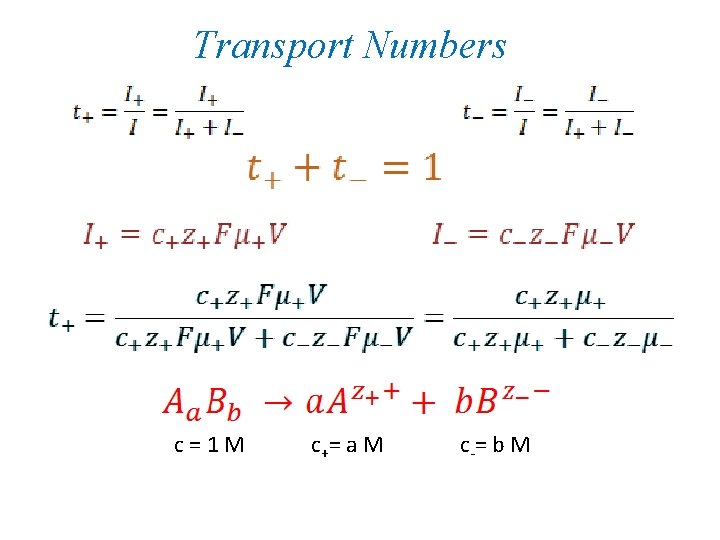
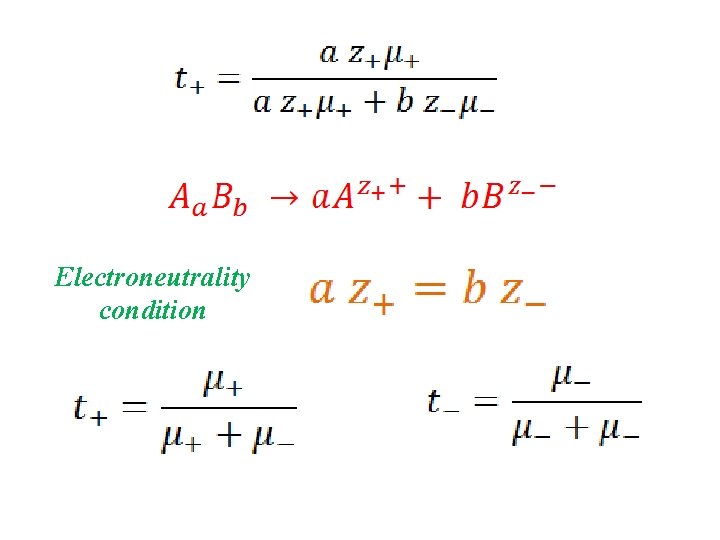
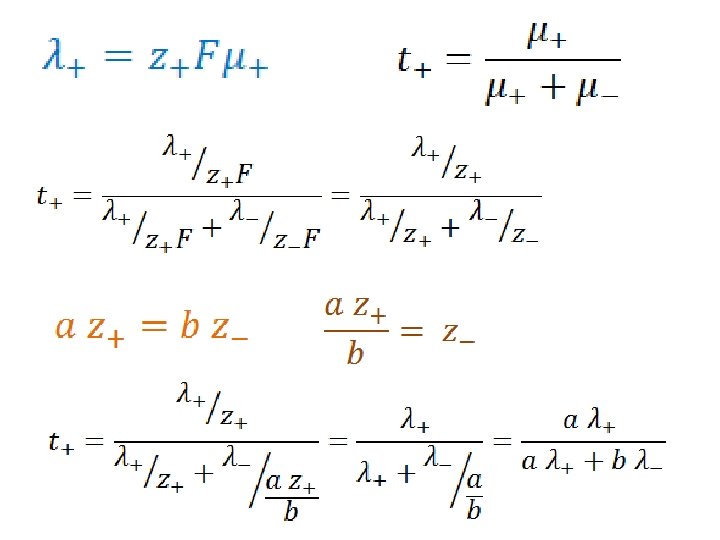
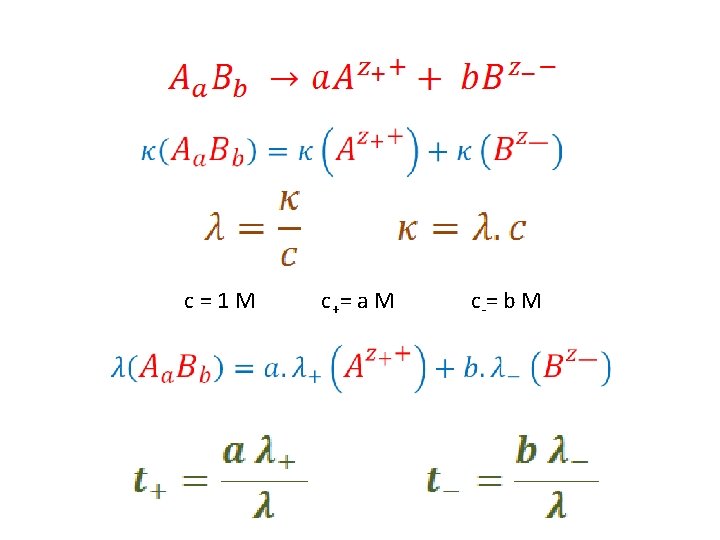
- Slides: 29

Ion Association Ion pair formation

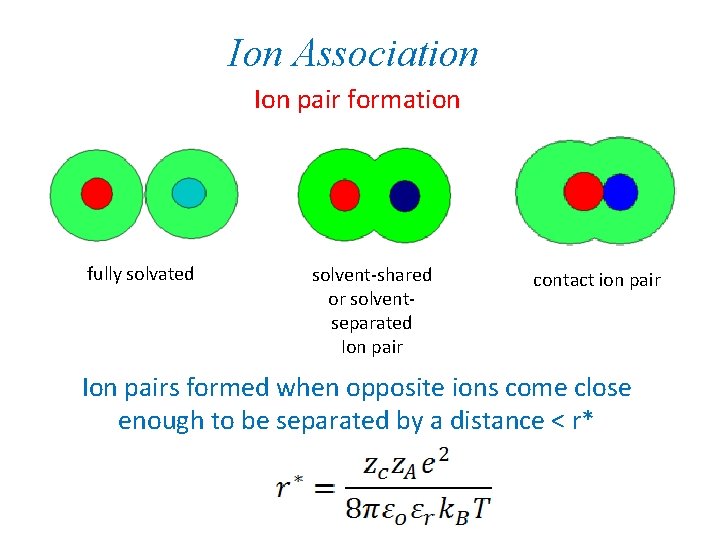
Ion Association Ion pair formation fully solvated solvent-shared or solventseparated Ion pair contact ion pair Ion pairs formed when opposite ions come close enough to be separated by a distance < r*

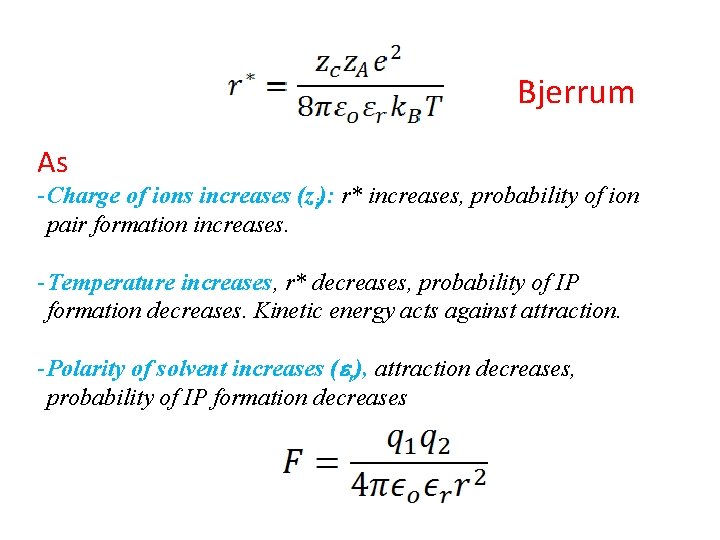
Bjerrum As - Charge of ions increases (zi): r* increases, probability of ion pair formation increases. - Temperature increases, r* decreases, probability of IP formation decreases. Kinetic energy acts against attraction. - Polarity of solvent increases (er), attraction decreases, probability of IP formation decreases

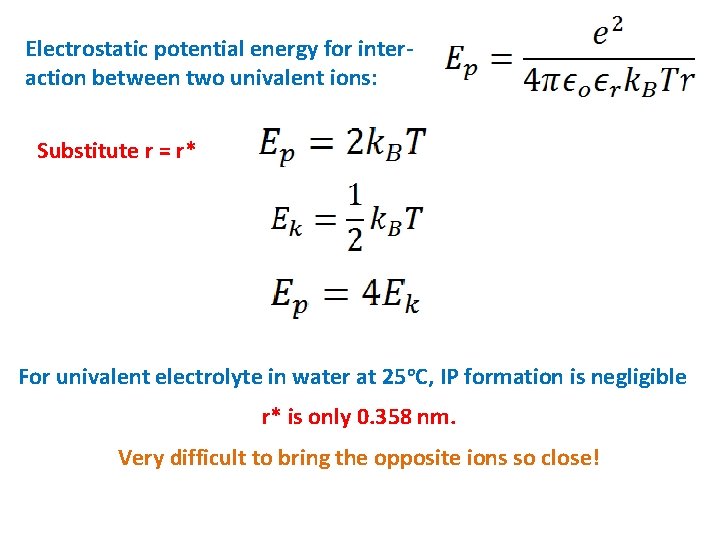
Electrostatic potential energy for interaction between two univalent ions: Substitute r = r* For univalent electrolyte in water at 25 o. C, IP formation is negligible r* is only 0. 358 nm. Very difficult to bring the opposite ions so close!


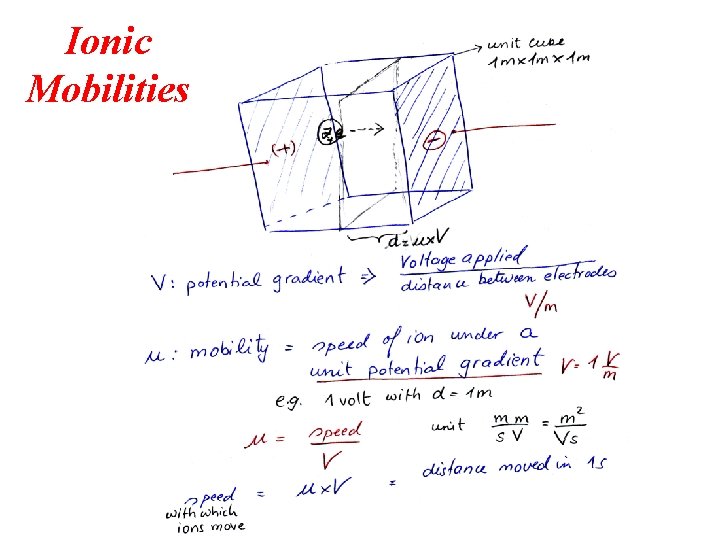
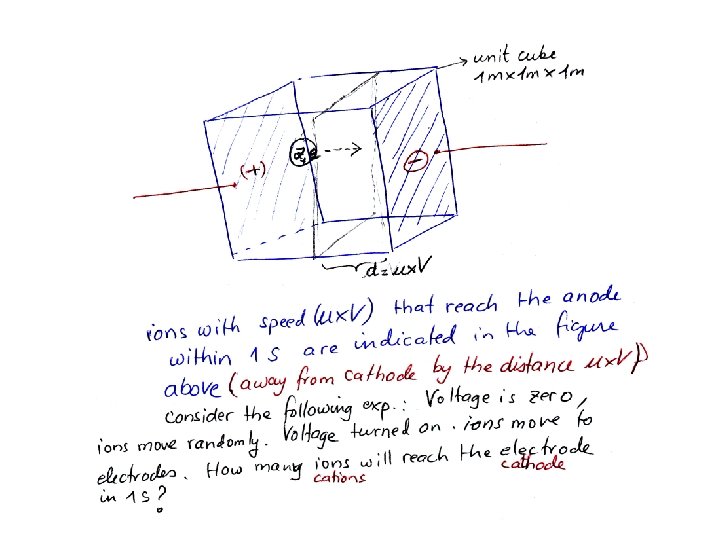
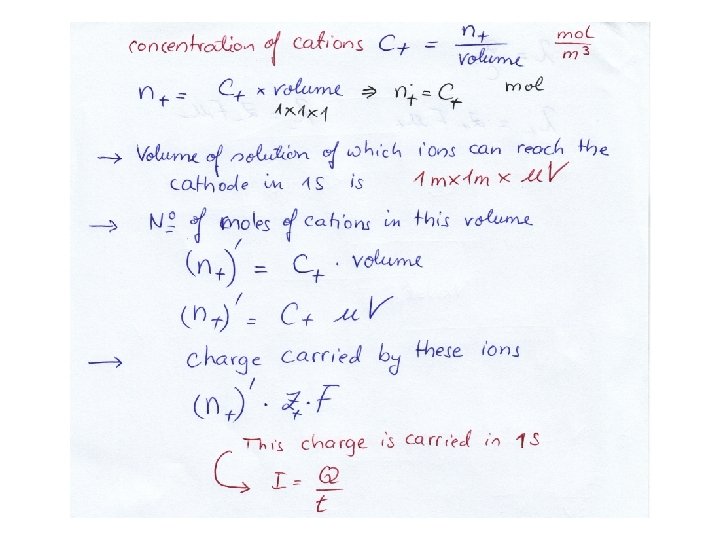
Ionic Mobilities




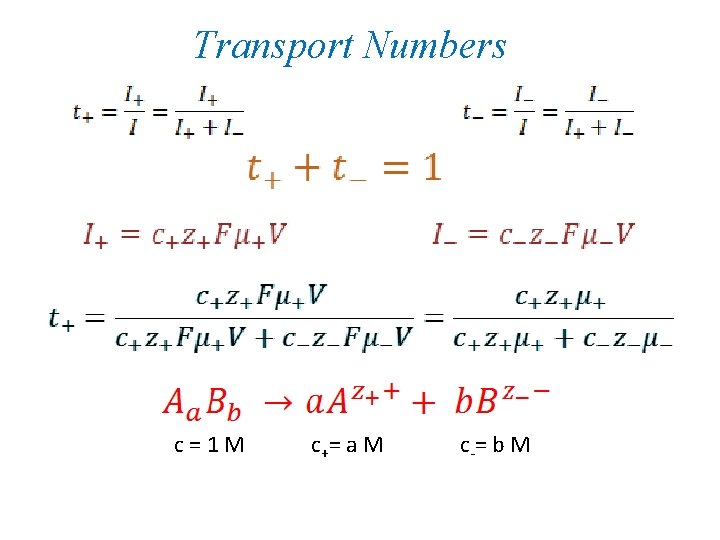
Transport Numbers c=1 M c += a M c -= b M
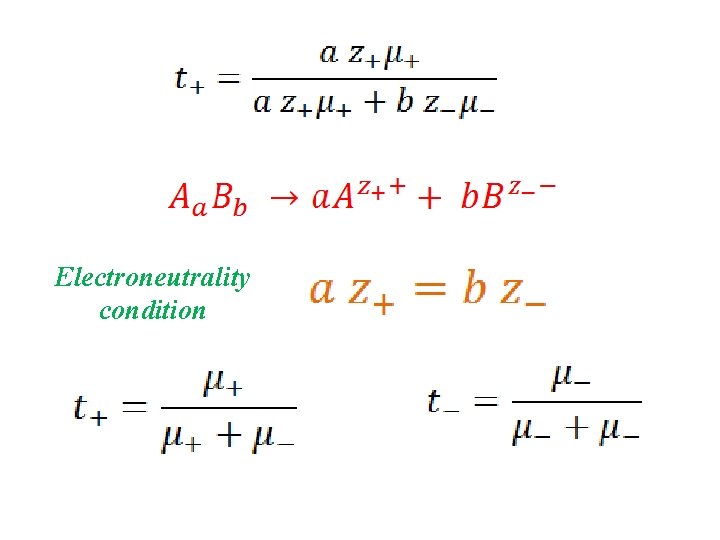
Electroneutrality condition
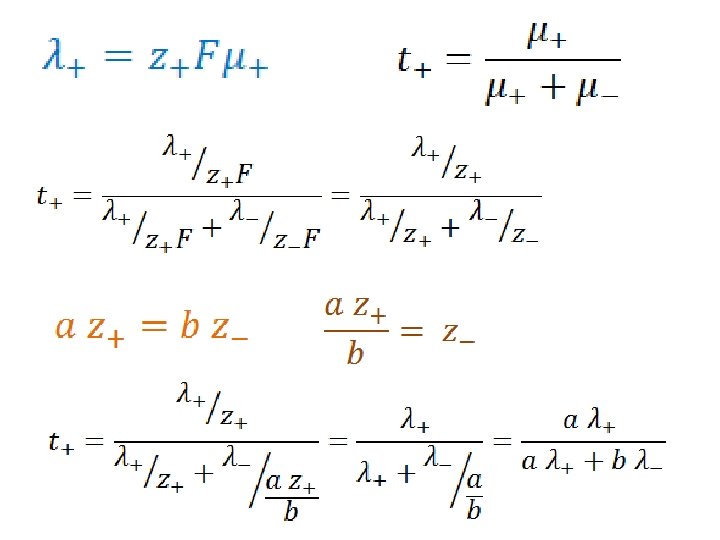
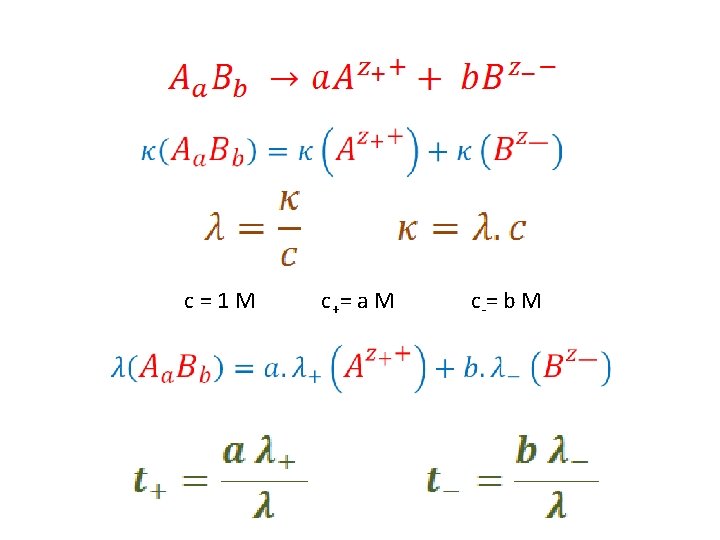
c=1 M c += a M c -= b M

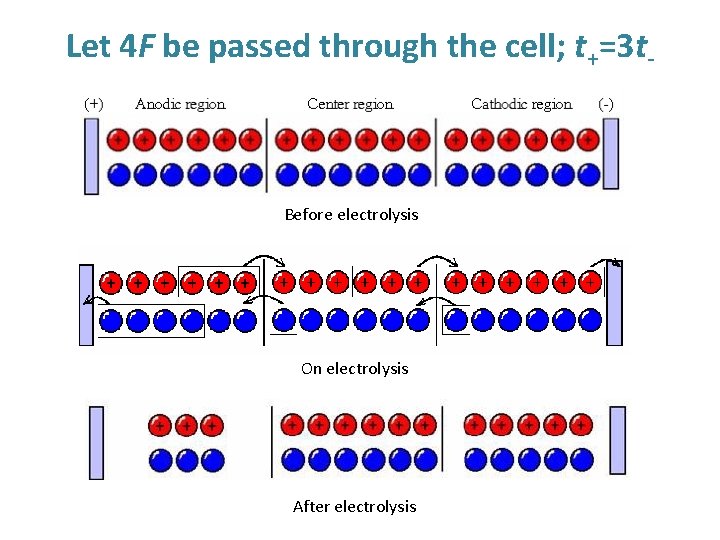
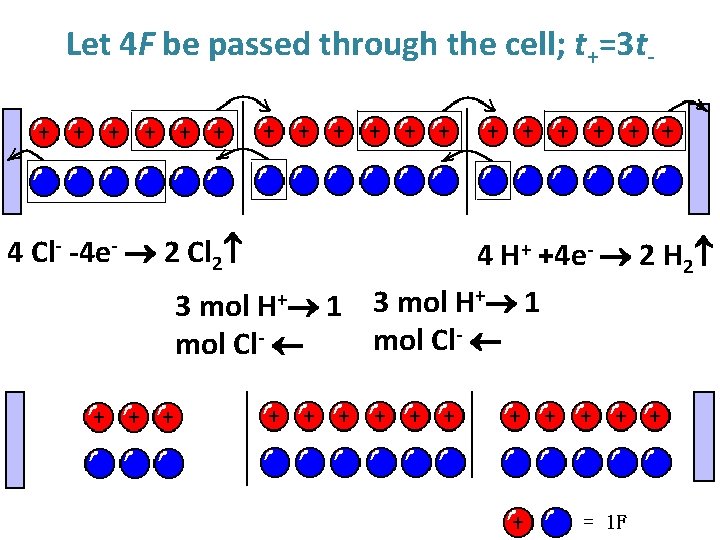
Let 4 F be passed through the cell; t+=3 t- Before electrolysis On electrolysis After electrolysis

Let 4 F be passed through the cell; t+=3 t- 4 Cl- -4 e- 2 Cl 2 4 H+ +4 e- 2 H 2 3 mol H+ 1 mol Cl-

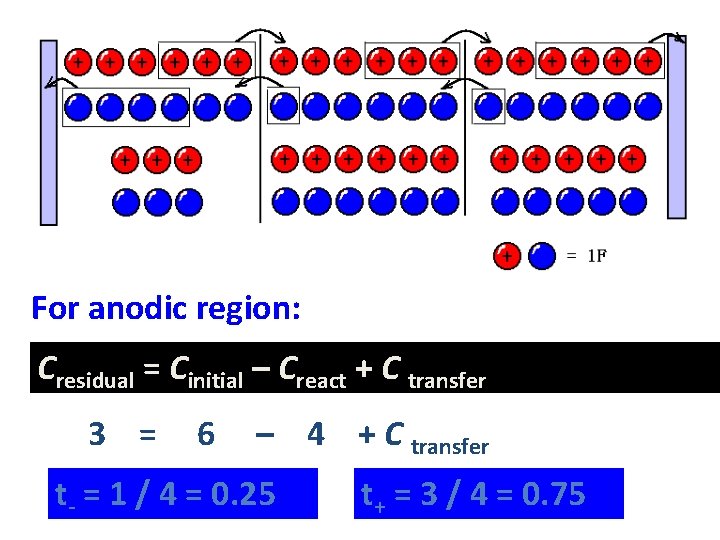
For anodic region: Cresidual = Cinitial – Creact + C transfer 3 = 6 – 4 + C transfer t- = 1 / 4 = 0. 25 t+ = 3 / 4 = 0. 75

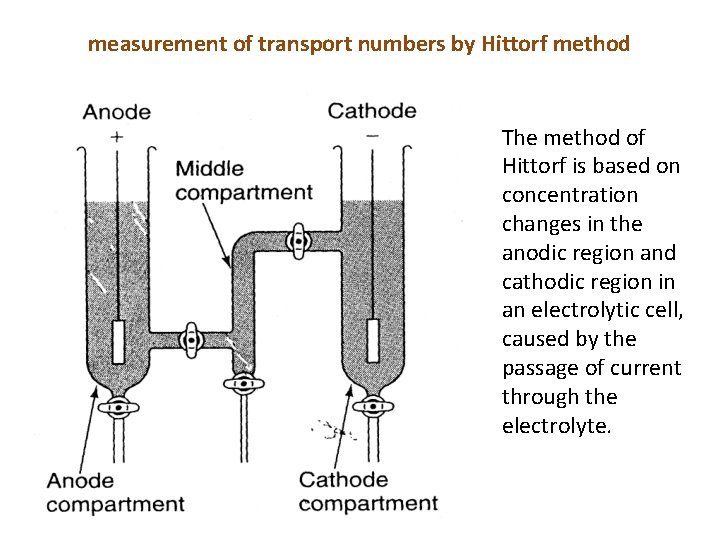
measurement of transport numbers by Hittorf method The method of Hittorf is based on concentration changes in the anodic region and cathodic region in an electrolytic cell, caused by the passage of current through the electrolyte.

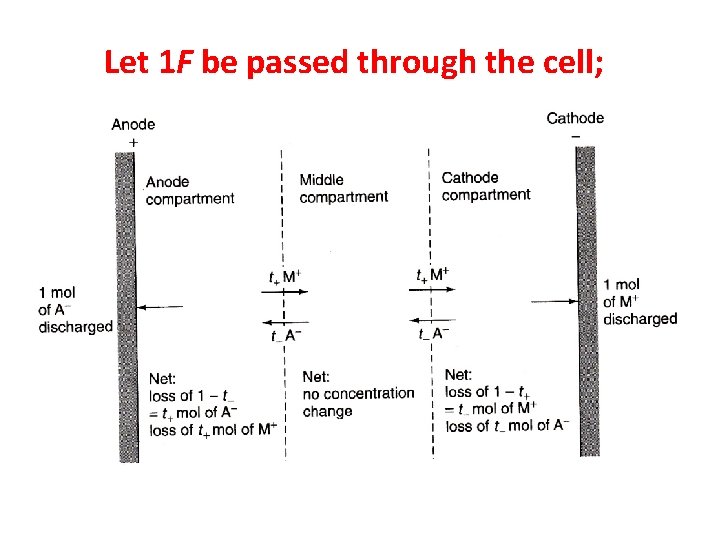
Let 1 F be passed through the cell;

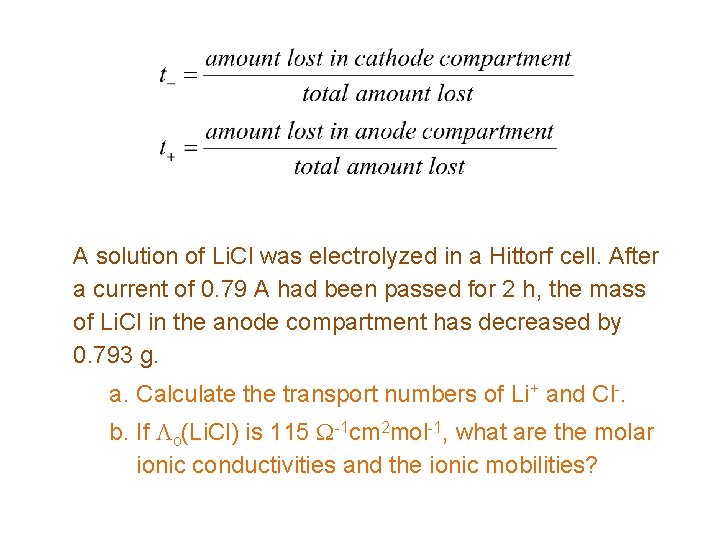
A solution of Li. Cl was electrolyzed in a Hittorf cell. After a current of 0. 79 A had been passed for 2 h, the mass of Li. Cl in the anode compartment has decreased by 0. 793 g. a. Calculate the transport numbers of Li+ and Cl-. b. If Lo(Li. Cl) is 115 W-1 cm 2 mol-1, what are the molar ionic conductivities and the ionic mobilities?

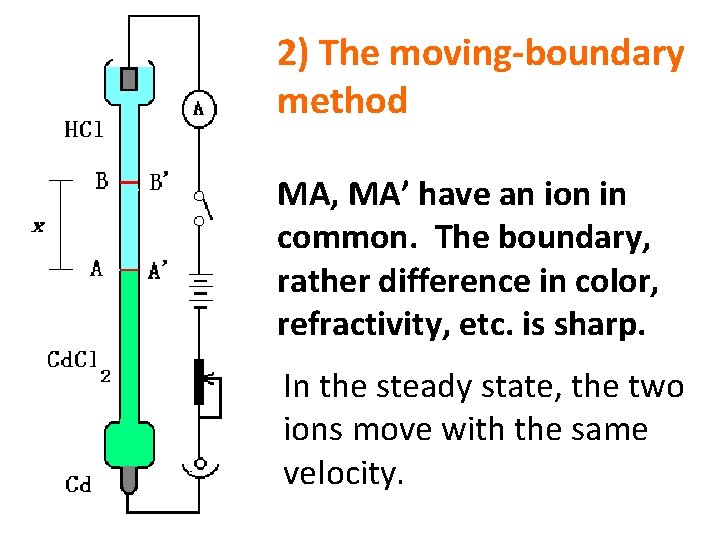
2) The moving-boundary method MA, MA’ have an ion in common. The boundary, rather difference in color, refractivity, etc. is sharp. In the steady state, the two ions move with the same velocity.

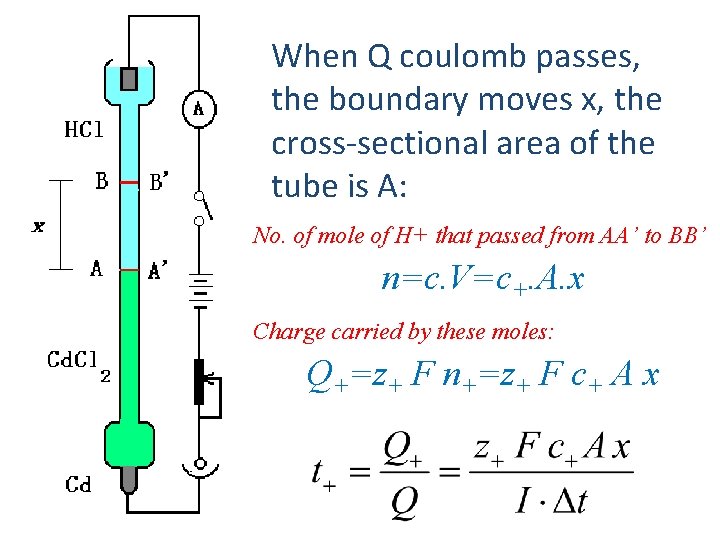
When Q coulomb passes, the boundary moves x, the cross-sectional area of the tube is A: No. of mole of H+ that passed from AA’ to BB’ n=c. V=c+. A. x Charge carried by these moles: Q+=z+ F n+=z+ F c+ A x

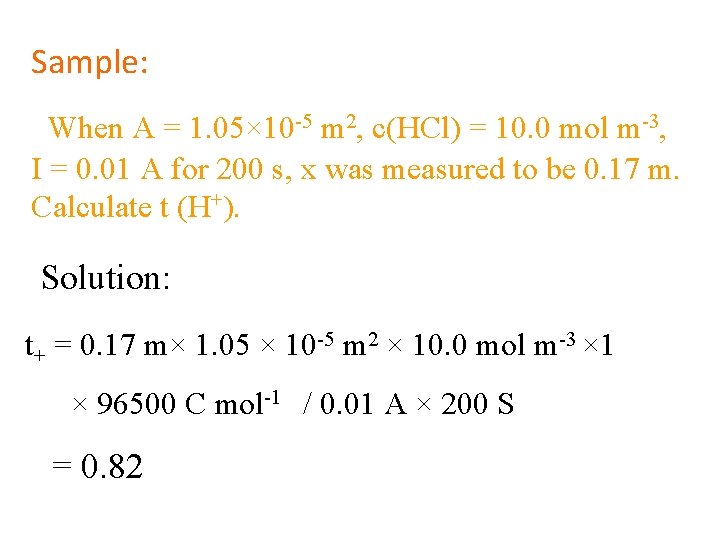
Sample: When A = 1. 05× 10 -5 m 2, c(HCl) = 10. 0 mol m-3, I = 0. 01 A for 200 s, x was measured to be 0. 17 m. Calculate t (H+). Solution: t+ = 0. 17 m× 1. 05 × 10 -5 m 2 × 10. 0 mol m-3 × 1 × 96500 C mol-1 / 0. 01 A × 200 S = 0. 82

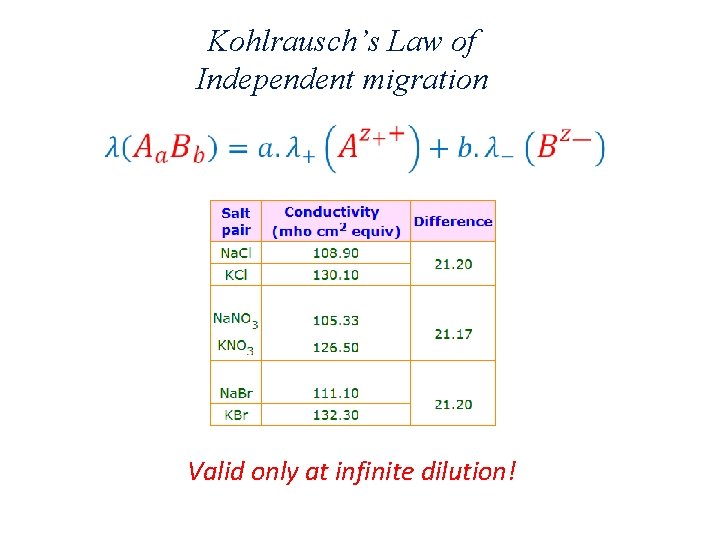
Kohlrausch’s Law of Independent migration Valid only at infinite dilution!


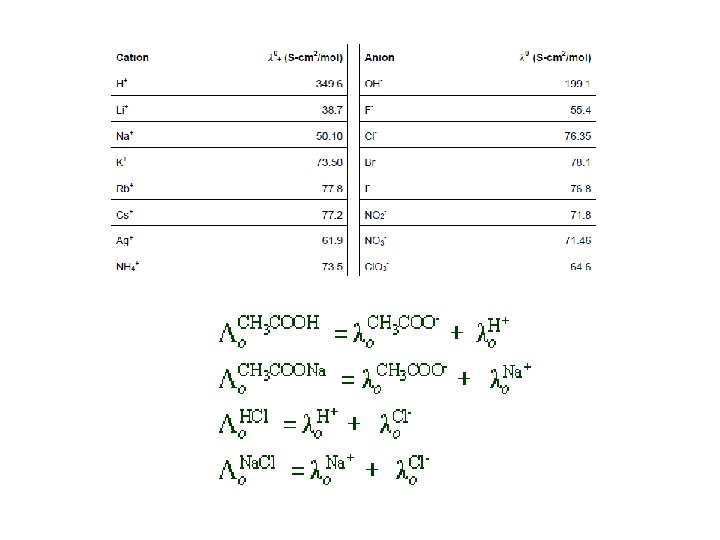
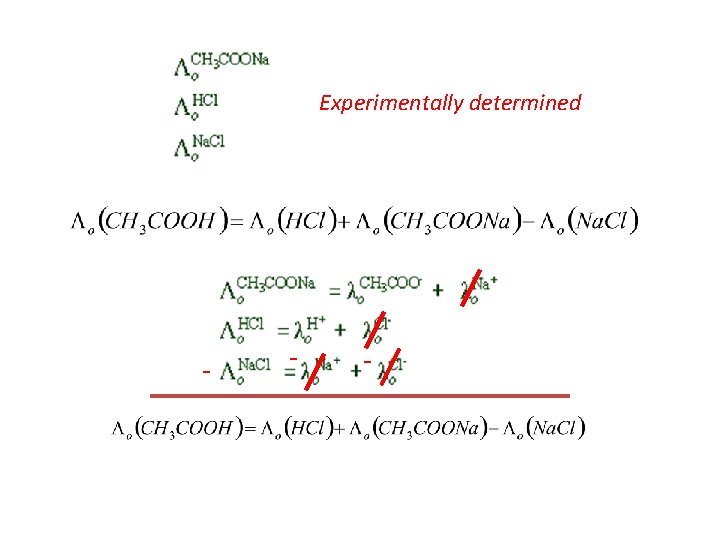
Experimentally determined - - -

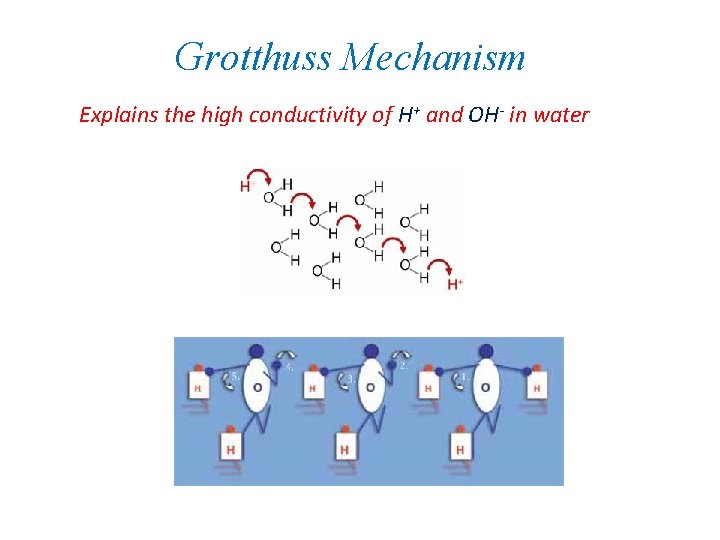
Grotthuss Mechanism Explains the high conductivity of H+ and OH- in water

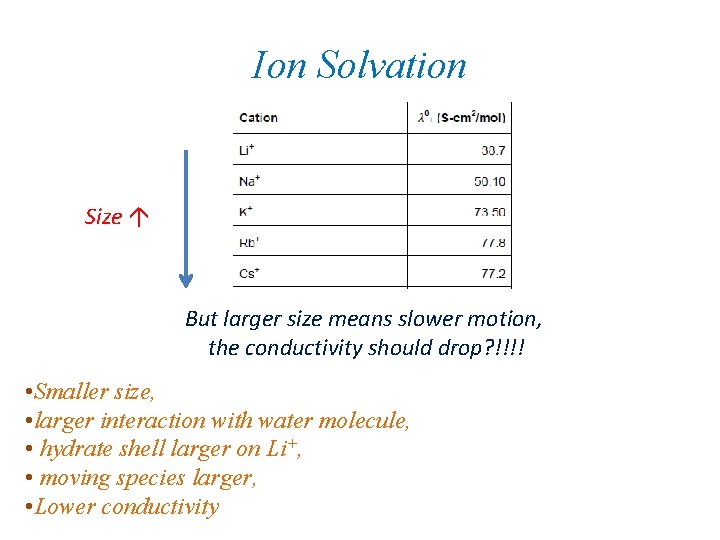
Ion Solvation Size But larger size means slower motion, the conductivity should drop? !!!! • Smaller size, • larger interaction with water molecule, • hydrate shell larger on Li+, • moving species larger, • Lower conductivity

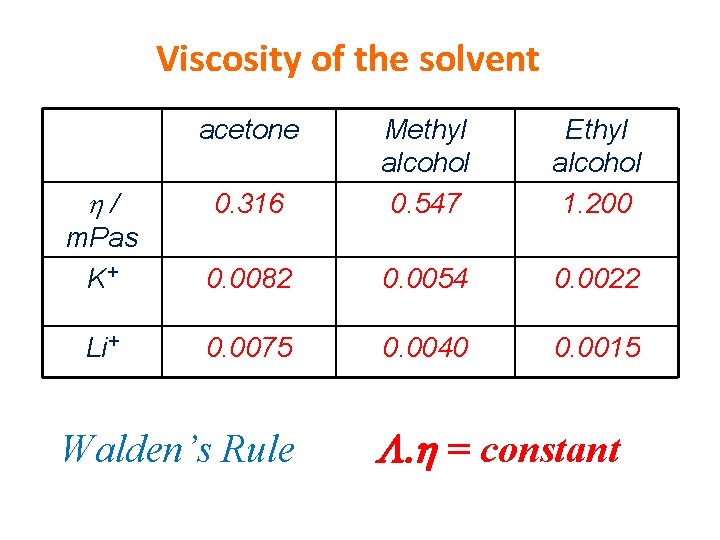
Viscosity of the solvent acetone / 0. 316 Methyl alcohol 0. 547 m. Pas K+ 0. 0082 0. 0054 0. 0022 Li+ 0. 0075 0. 0040 0. 0015 Walden’s Rule Ethyl alcohol 1. 200 L. h = constant