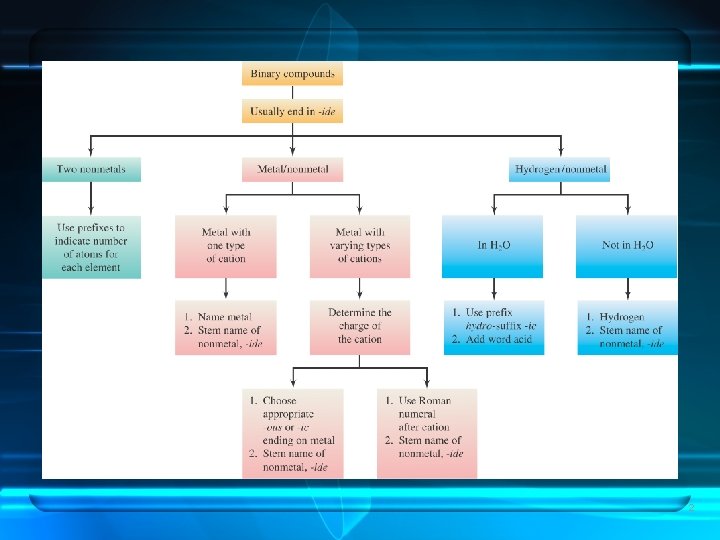
CHEM 105 Naming Inorganic Compounds Containing Polyatomic Ions
























- Slides: 37

CHEM 105 Naming Inorganic Compounds Containing Polyatomic Ions

2

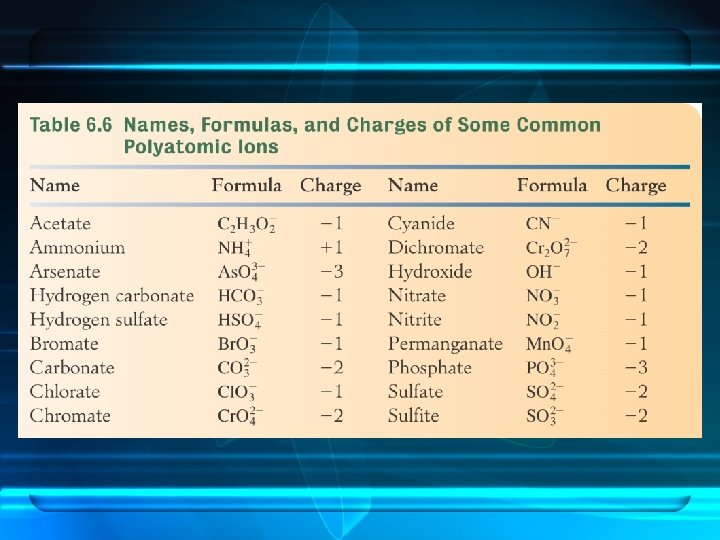
A polyatomic ion is an ion that contains two or more elements.

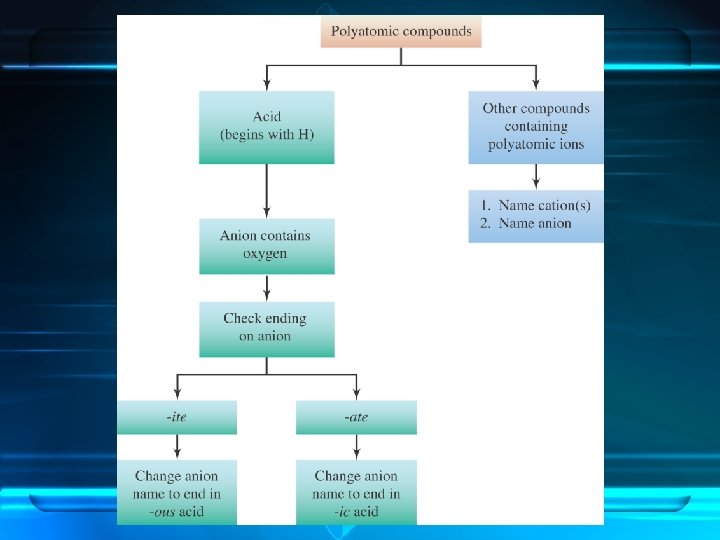
• Compounds containing polyatomic ions are composed of three or more elements. • They usually consist of one or more cations combined with a negative polyatomic ion.

• When naming a compound containing a polyatomic ion, name the cation first and then name the anion.

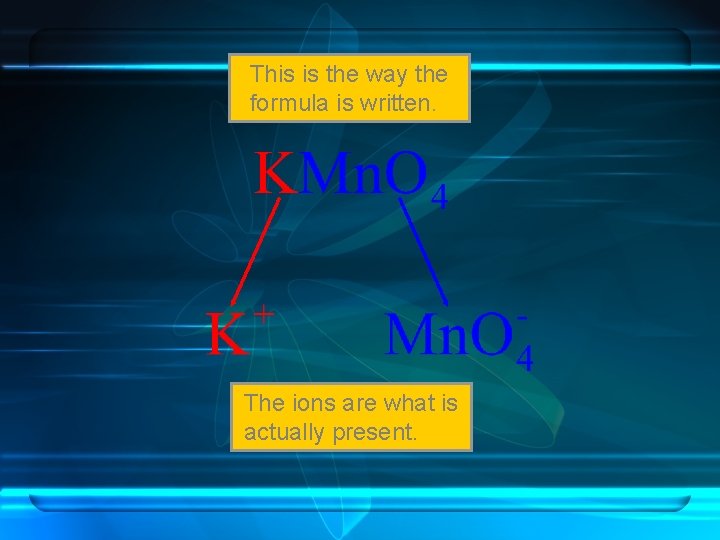
This is the way the formula is written. The ions are what is actually present.

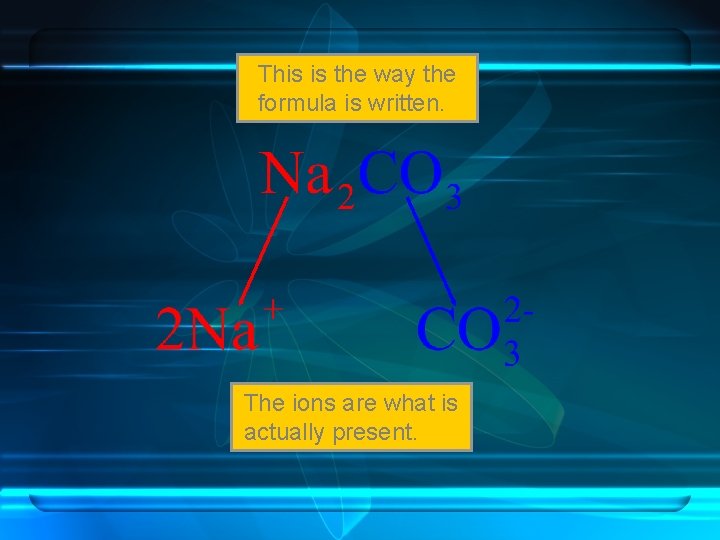
This is the way the formula is written. The ions are what is actually present.

Prefixes and Suffixes Elements that Form More than One Polyatomic Ion with Oxygen

Anions ending in -ate always contain more oxygen than ions ending in -ite. nitrite nitrate

Anions ending in -ate always contain more oxygen than ions ending in -ite. phosphite phosphate

Anions ending in -ate always contain more oxygen than ions ending in -ite. sulfite sulfate -ate and –ite do not indicate the number of oxygen atoms.

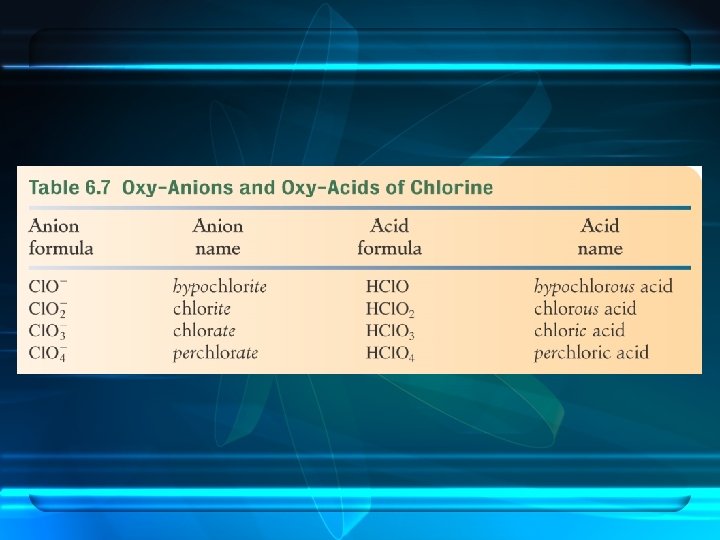
per- denotes anions with more oxygen than the -ate form. chlorate perchlorate

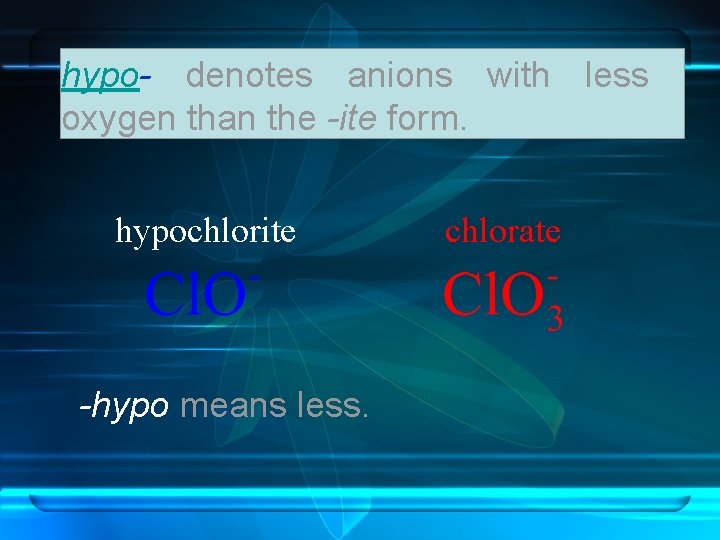
hypo- denotes anions with less oxygen than the -ite form. hypochlorite -hypo means less. chlorate


Four ions do not use the –ate/ite system. hydroxide cyanide hydrogen sulfide peroxide

There are three common positively charged polyatomic ions. mercury(I) hydronium ammonium


Acids

Oxy-acids contain hydrogen, oxygen and one other element. • The other element is usually a nonmetal, but it can be a metal. • Its first element is hydrogen. • Its remaining elements include oxygen and form a polyatomic ion.

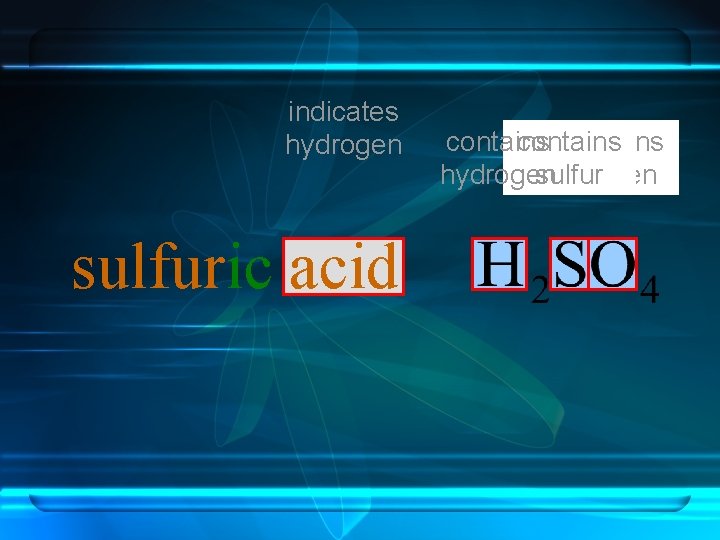
Hydrogen in an oxy-acid is not expressed in the acid name. The word acid in the name indicates the presence of hydrogen.

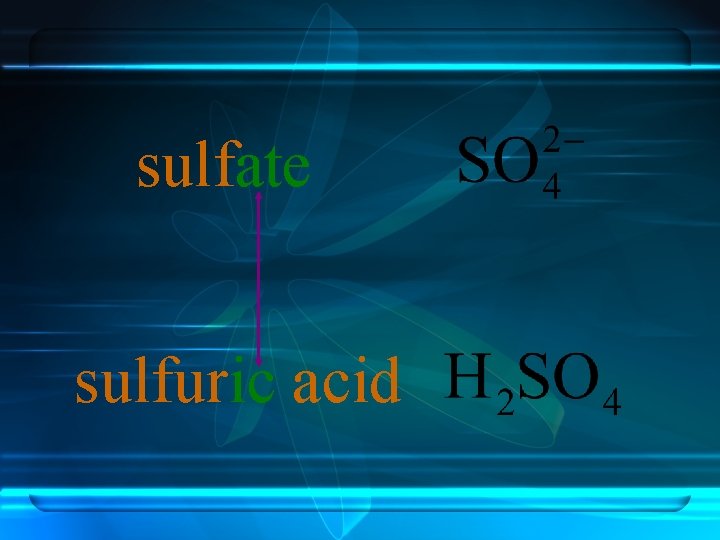
indicates hydrogen sulfuric acid contains hydrogen sulfur oxygen

Anions ending in -ate always contain more oxygen than ions ending in -ite. phosphite phosphate

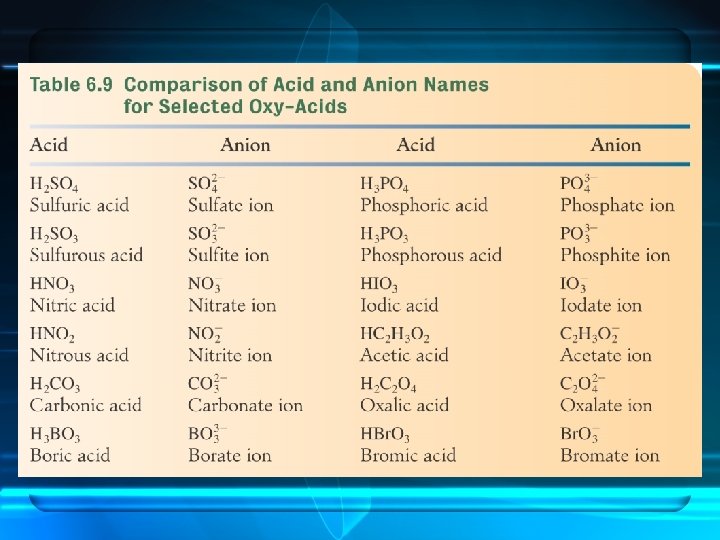
Naming the Acid Based on the Name of the Polyatomic Ion Ending of Acid ite ous ate ic less oxygen more oxygen

Examples

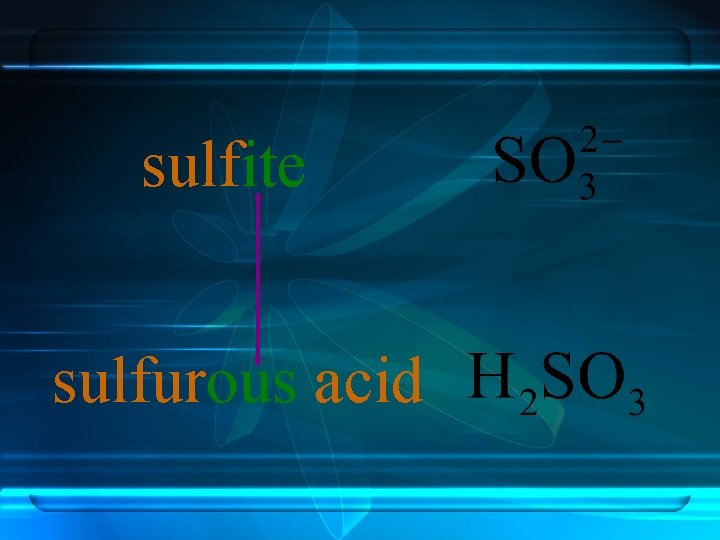
sulfite sulfurous acid

sulfate sulfuric acid

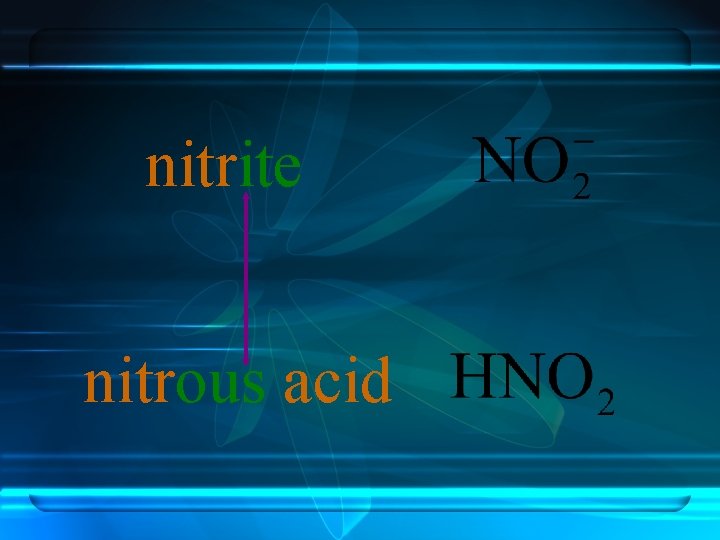
nitrite nitrous acid

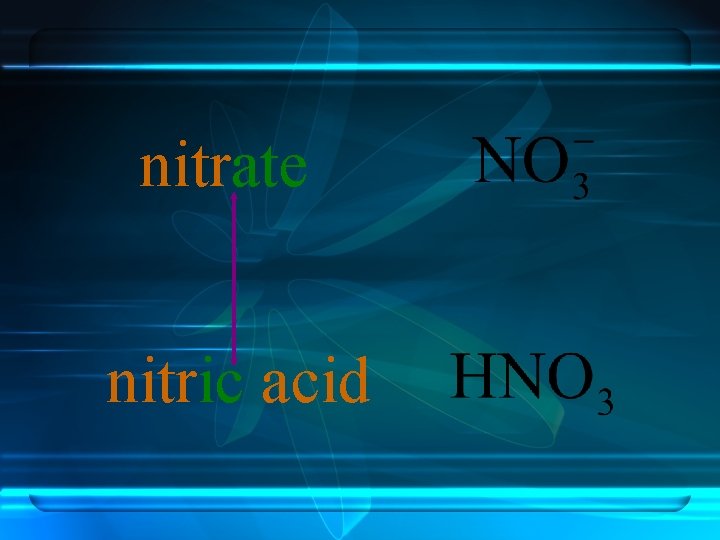
nitrate nitric acid



Writing Formulas From Names of Compounds

A chemical compound must have a net charge of zero.

If the compound contains ions, then the charges on all of the ions must add to zero. ryan likes boys

Write the formula of calcium chloride. Step 1. Write down the formulas of the ions. Ca 2+ Cl. Step 2. Combine the smallest numbers of Ca 2+ - so that the sum of the charges and Cl The cation Theisanion is equals written first. zero. second. (Ca 2+) + 2(Cl-) = 0 (2+) + 2(1 -) = 0 The lowest common multiple The correct formula is Ca. Cl 2 of +2 and – 1 is 2

Write the formula of barium phosphide. Step 1. Write down the formulas of the ions. Ba 2+ P 3 Step 2. Combine the smallest numbers of Ba 2+ 3 - so that the sum of the charges and P The cation The anion is is equals zero. written first. second. 3(Ba 2+) + 2(P 3 -) = 0 3(2+) + 2(3 -) = 0 The lowest common multiple The correct formula is Ba 3 P 2 of +2 and – 3 is 6

Write the formula of magnesium oxide. Step 1. Write down the formulas of the ions. Mg 2+ O 2 Step 2. Combine the smallest numbers of Mg 2+ and O 2 - so that the sum of the charges equals zero. (Mg 2+) + (O 2 -) = 0 (2+) + (2 -) = 0 The lowest common multiple The correct formula is Mg. O of +2 and – 2 is 1
