Ch 15 and 6 Polyatomic Ions Polyatomic Ions





- Slides: 16

Ch. 15 and 6 Polyatomic Ions

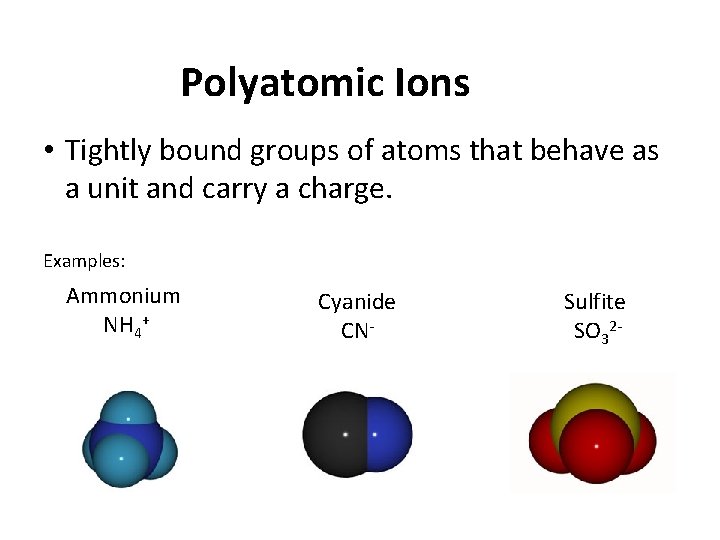
Polyatomic Ions • Tightly bound groups of atoms that behave as a unit and carry a charge. Examples: Ammonium NH 4+ Cyanide CN- Sulfite SO 32 -

Common Polyatomic Ions with a -1 charge • • Cl. O-1 hypochlorite Cl. O 2 -1 chlorite Cl. O 3 -1 chlorate Cl. O 4 -1 perchlorate CN-1 cyanide Mn. O 4 -1 permanganate NO 3 -1 nitrate NO 2 -1 nitrite

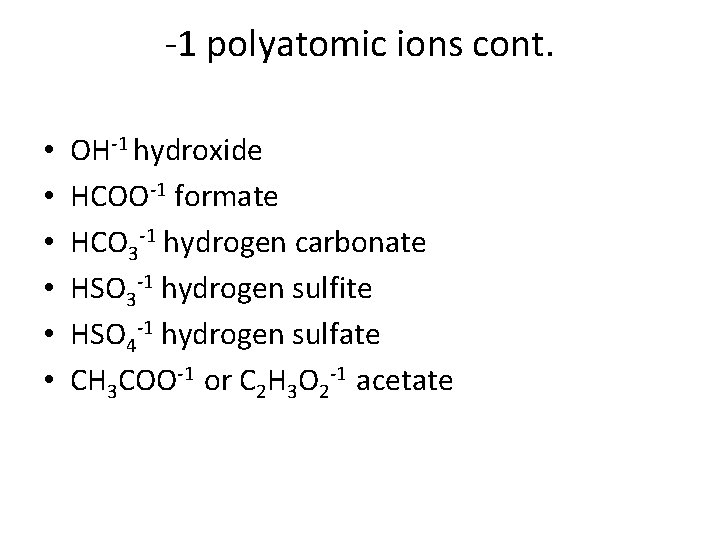
-1 polyatomic ions cont. • • • OH-1 hydroxide HCOO-1 formate HCO 3 -1 hydrogen carbonate HSO 3 -1 hydrogen sulfite HSO 4 -1 hydrogen sulfate CH 3 COO-1 or C 2 H 3 O 2 -1 acetate

-2 polyatomic ions • • • C 2 O 4 -2 oxalate CO 3 -2 carbonate Cr 2 O 7 -2 dichromate Cr. O 4 -2 chromate SO 4 -2 sulfate SO 3 -2 sulfite

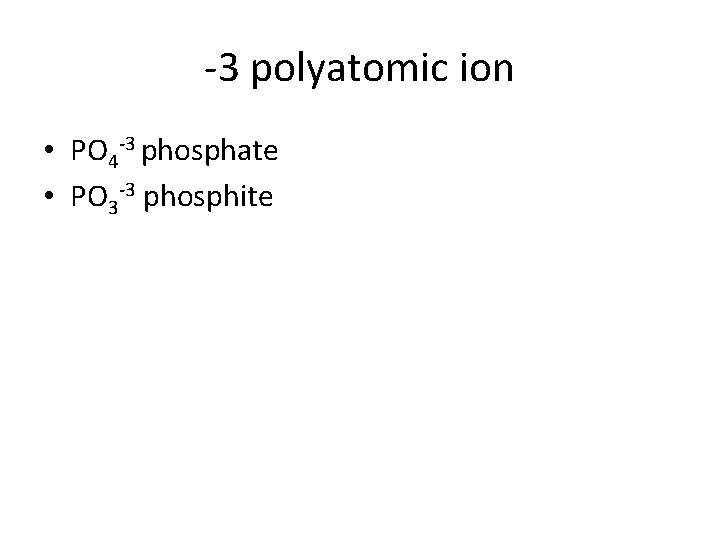
-3 polyatomic ion • PO 4 -3 phosphate • PO 3 -3 phosphite

+1 polyatomic ions • NH 4+1 ammonium • H 3 O+1 hydronium

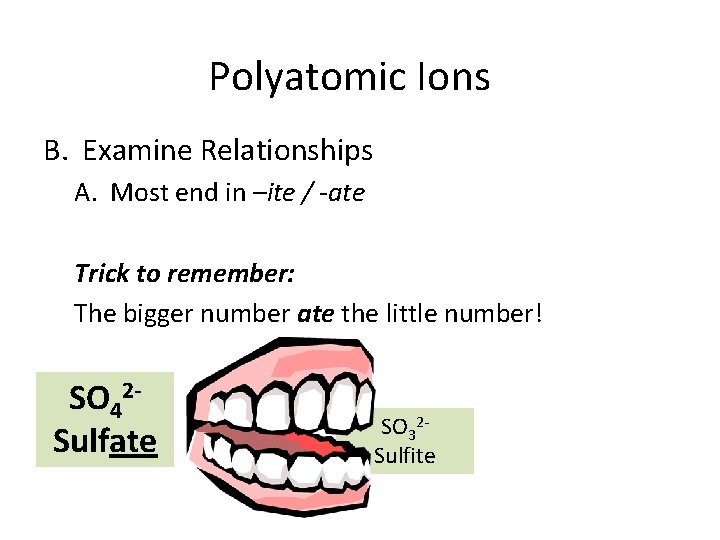
Polyatomic Ions B. Examine Relationships A. Most end in –ite / -ate Trick to remember: The bigger number ate the little number! SO 42 Sulfate SO 32 Sulfite

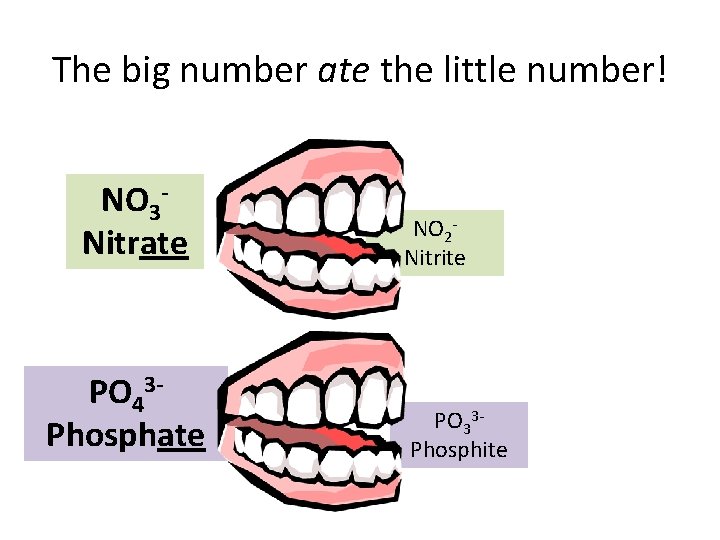
The big number ate the little number! NO 3 Nitrate PO 43 Phosphate NO 2 Nitrite PO 33 Phosphite

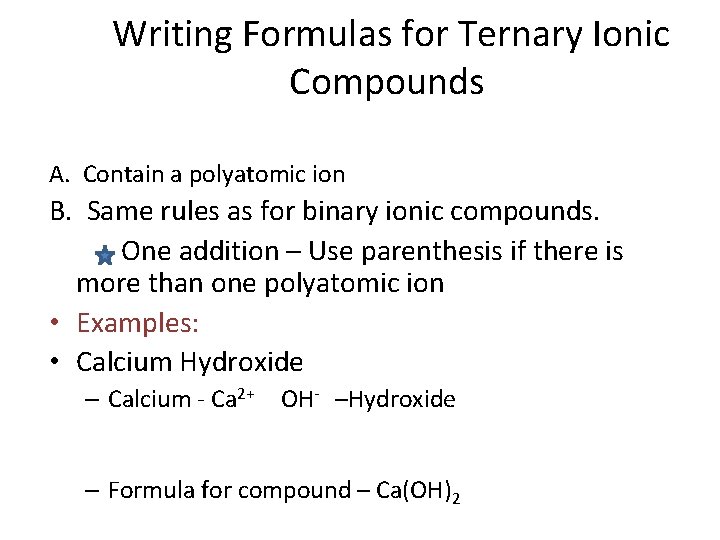
Writing Formulas for Ternary Ionic Compounds A. Contain a polyatomic ion B. Same rules as for binary ionic compounds. One addition – Use parenthesis if there is more than one polyatomic ion • Examples: • Calcium Hydroxide – Calcium - Ca 2+ OH- –Hydroxide – Formula for compound – Ca(OH)2

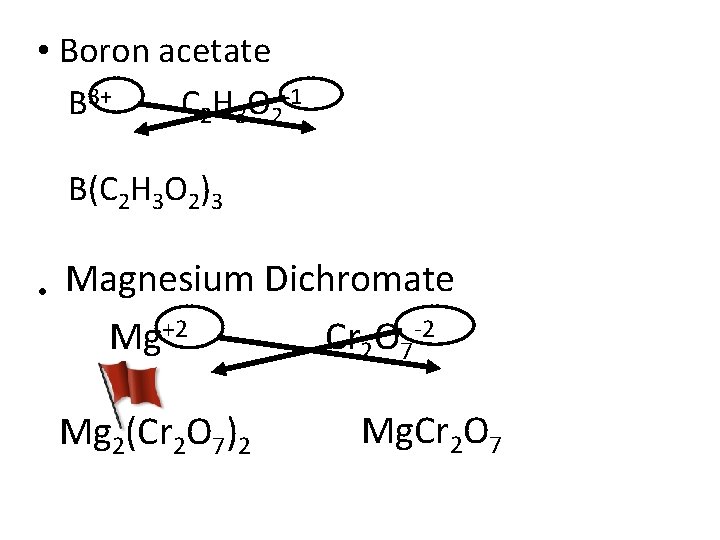
• Boron acetate B 3+ C 2 H 3 O 2 -1 B(C 2 H 3 O 2)3 • Magnesium Dichromate Mg+2 Cr 2 O 7 -2 Mg 2(Cr 2 O 7)2 Mg. Cr 2 O 7

Examples (cont. ) • Magnesium Nitrate – Magnesium – Mg 2+ – Nitrate - ? ? – Write the formula for the compound

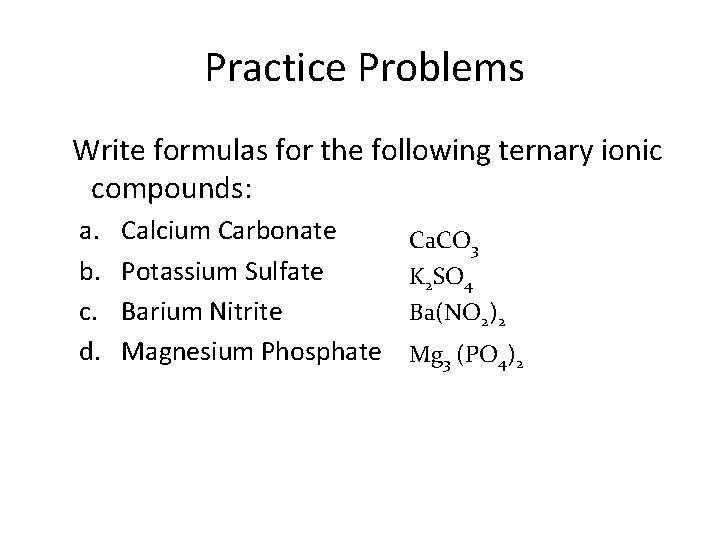
Practice Problems Write formulas for the following ternary ionic compounds: a. b. c. d. Calcium Carbonate Potassium Sulfate Barium Nitrite Magnesium Phosphate Ca. CO 3 K 2 SO 4 Ba(NO 2)2 Mg 3 (PO 4)2

Naming Ternary Ionic Compounds • Same rules as for naming binary ionic compounds: – Name the cation – Use a Roman numeral if necessary – Name the anion – Do not use the word “ion” in the compound name

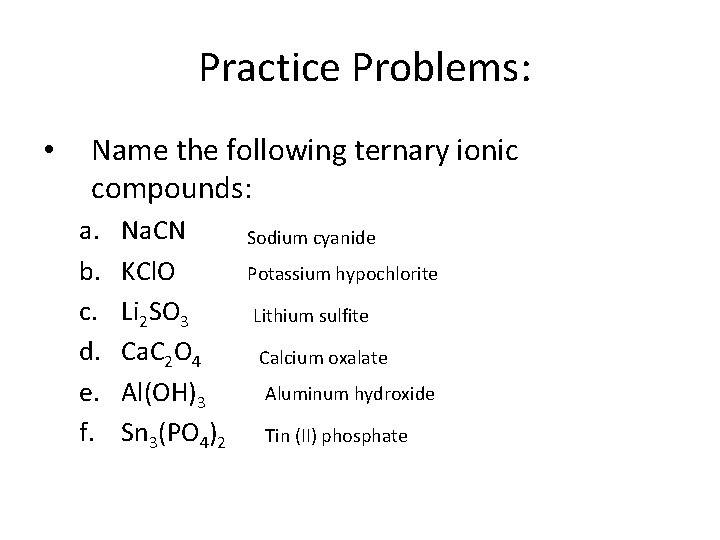
Practice Problems: • Name the following ternary ionic compounds: a. b. c. d. e. f. Na. CN KCl. O Li 2 SO 3 Ca. C 2 O 4 Al(OH)3 Sn 3(PO 4)2 Sodium cyanide Potassium hypochlorite Lithium sulfite Calcium oxalate Aluminum hydroxide Tin (II) phosphate

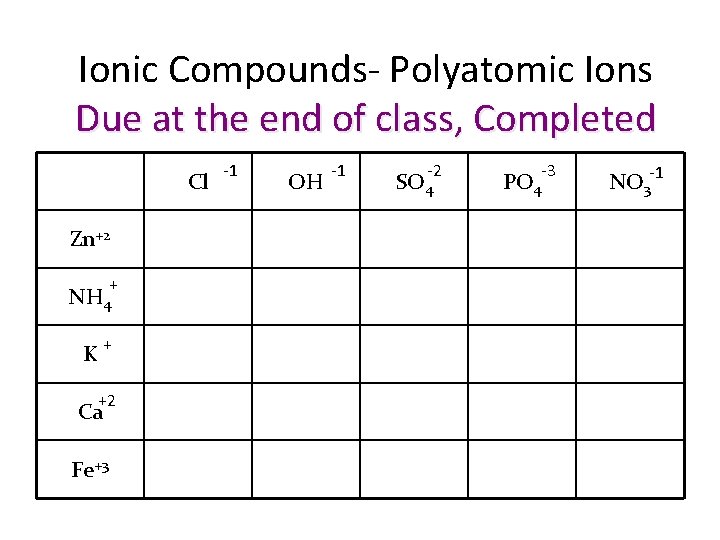
Ionic Compounds- Polyatomic Ions Due at the end of class, Completed Cl Zn+2 + NH 4 + K +2 Ca Fe+3 -1 OH -1 -2 SO 4 PO 4 -3 -1 NO 3