INORGANIC COMPOUNDS Inorganic Compounds compounds that do NOT






- Slides: 16

INORGANIC COMPOUNDS

Inorganic Compounds – compounds that do NOT contain carbon There are two major inorganic compounds that are important to living things 1. ATP 2. Water

1. ATP Compound that stores and releases energy in the cell Energy released from food is stored in ATP, where it can be quickly and easily used by the cell Stands for Adenosine Triphosphate All living things need energy to carry on their life activities. This is obtained from the chemical energy stored in their food.

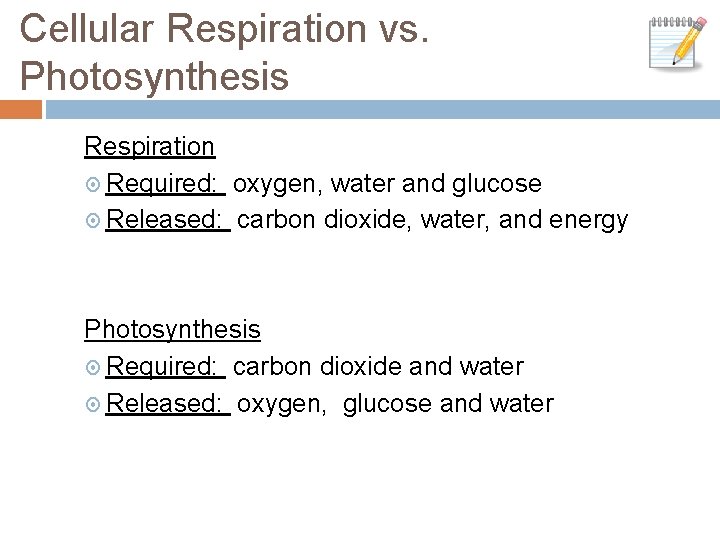
Cellular Respiration vs. Photosynthesis Respiration Required: oxygen, water and glucose Released: carbon dioxide, water, and energy Photosynthesis Required: carbon dioxide and water Released: oxygen, glucose and water

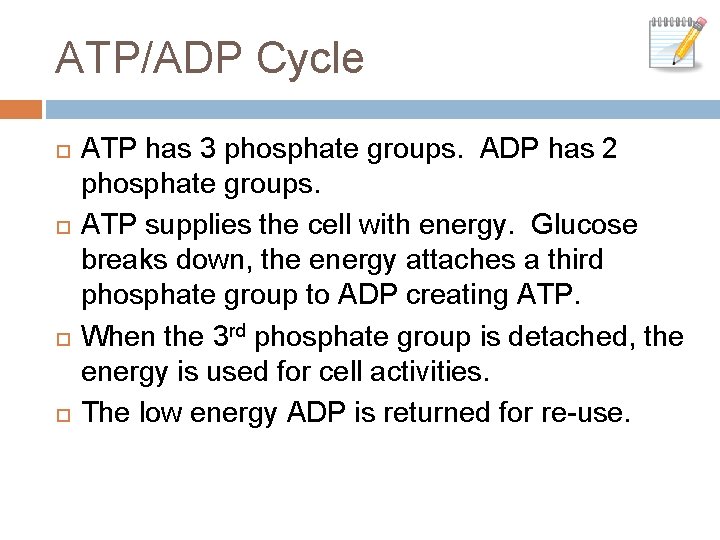
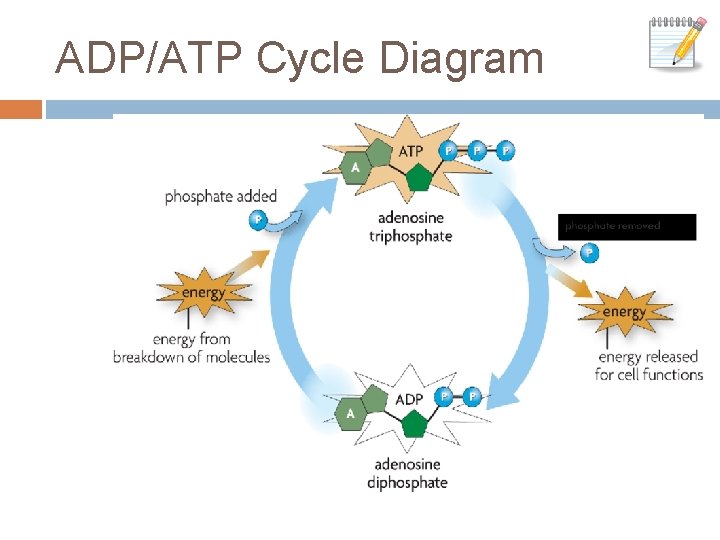
ATP/ADP Cycle ATP has 3 phosphate groups. ADP has 2 phosphate groups. ATP supplies the cell with energy. Glucose breaks down, the energy attaches a third phosphate group to ADP creating ATP. When the 3 rd phosphate group is detached, the energy is used for cell activities. The low energy ADP is returned for re-use.

ADP/ATP Cycle Diagram

2. WATER

Facts about water 70% of your body is water Body’s cells are filled with water Most cellular events must take place in water Water helps nutrients move in and out of cells

PROPERTIES OF WATER

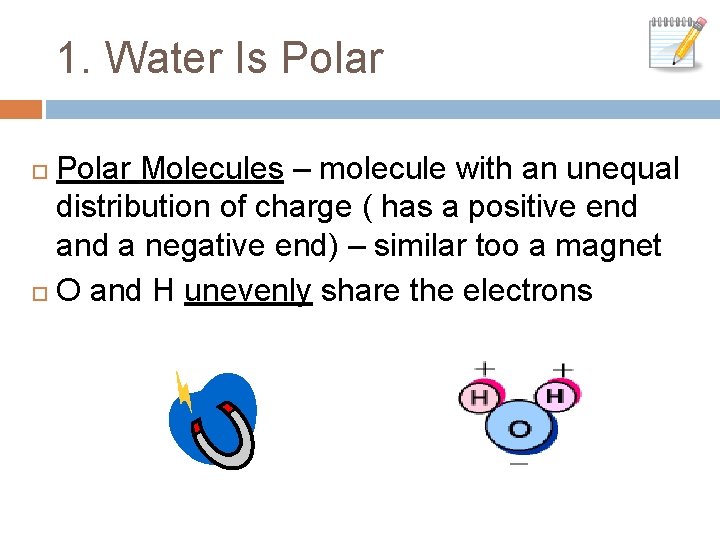
1. Water Is Polar Molecules – molecule with an unequal distribution of charge ( has a positive end a negative end) – similar too a magnet O and H unevenly share the electrons

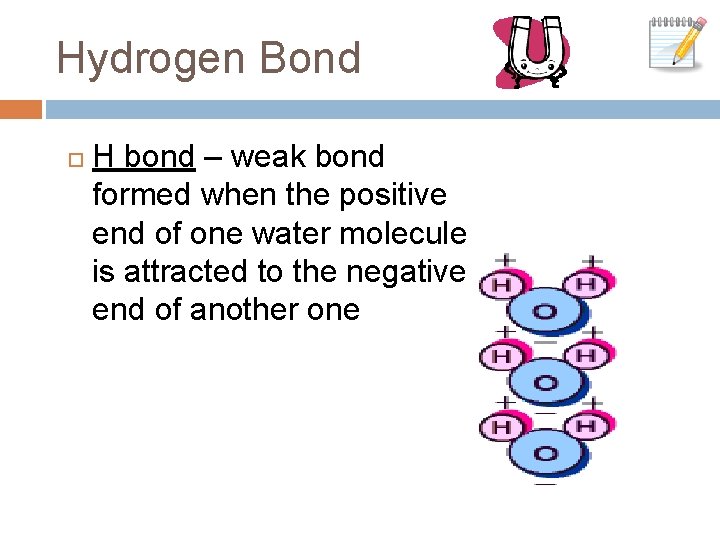
Hydrogen Bond H bond – weak bond formed when the positive end of one water molecule is attracted to the negative end of another one

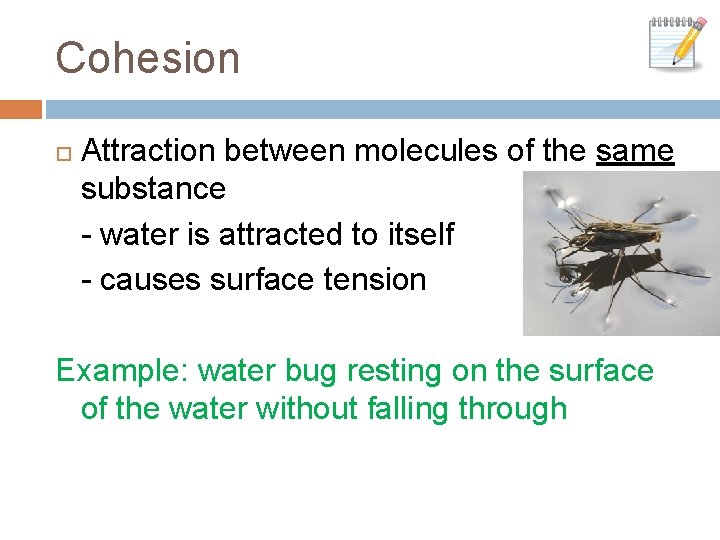
Cohesion Attraction between molecules of the same substance - water is attracted to itself - causes surface tension Example: water bug resting on the surface of the water without falling through

Adhesion Attraction between molecules of different substances - water has an attraction to other polar molecules ex: Capillary action – allows the tops of plants to get water from the soil ex: dissolving solutes – water molecules stick to other molecules allows the water to dissolve the substance

2. Water Resists Temperature Change Water requires more energy to increase its temperature than other liquids Water loses a lot of energy when it cools Helps prevent large temperature changes in organisms

3. Water Expands When it Freezes Ice is less dense than water (ice floats on water) Bottoms of lakes and ponds won’t freeze Water gets into the most stable configuration when it freezes

4. Water is a “Universal Solvent” Most other polar substances are dissolved easily in water Water is poor at dissolving non polar substances Water is known as a “universal solvent” but its name is not entirely accurate It dissolves more substances than any other liquid It cannot dissolve all substances an example