Classes of Compounds Inorganic compounds Water salts and




![Acid-Base Concentration • Acid solutions contain [H+] • As [H+] increases, acidity increases • Acid-Base Concentration • Acid solutions contain [H+] • As [H+] increases, acidity increases •](https://slidetodoc.com/presentation_image_h2/87619f5eccf050dfa6a50fa357333134/image-10.jpg)
![p. H: Acid-Base Concentration • p. H = the negative logarithm of [H+] in p. H: Acid-Base Concentration • p. H = the negative logarithm of [H+] in](https://slidetodoc.com/presentation_image_h2/87619f5eccf050dfa6a50fa357333134/image-11.jpg)
![p. H: Acid-Base Concentration • Acidic solutions • [H+], p. H • Acidic p. p. H: Acid-Base Concentration • Acidic solutions • [H+], p. H • Acidic p.](https://slidetodoc.com/presentation_image_h2/87619f5eccf050dfa6a50fa357333134/image-12.jpg)
![Concentration (moles/liter) Examples [OH–] [H+] p. H 100 10– 14 14 1 M Sodium Concentration (moles/liter) Examples [OH–] [H+] p. H 100 10– 14 14 1 M Sodium](https://slidetodoc.com/presentation_image_h2/87619f5eccf050dfa6a50fa357333134/image-13.jpg)

![Carbohydrates • Sugars and starches • Contain C, H, and O [(CH 20)n] • Carbohydrates • Sugars and starches • Contain C, H, and O [(CH 20)n] •](https://slidetodoc.com/presentation_image_h2/87619f5eccf050dfa6a50fa357333134/image-19.jpg)



- Slides: 26

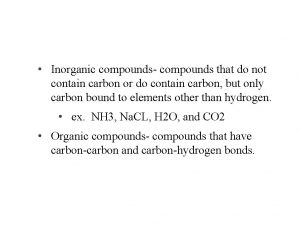
Classes of Compounds • Inorganic compounds • Water, salts, and many acids and bases • Do not contain carbon • Organic compounds • Carbohydrates, fats, proteins, and nucleic acids • Contain carbon, usually large, and are covalently bonded

Water • 60%– 80% of the volume of living cells • Most important inorganic compound in living organisms because of its properties

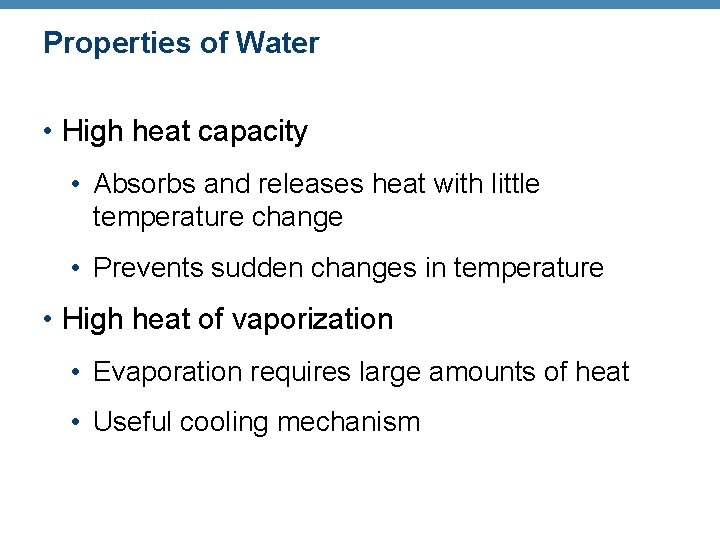
Properties of Water • High heat capacity • Absorbs and releases heat with little temperature change • Prevents sudden changes in temperature • High heat of vaporization • Evaporation requires large amounts of heat • Useful cooling mechanism

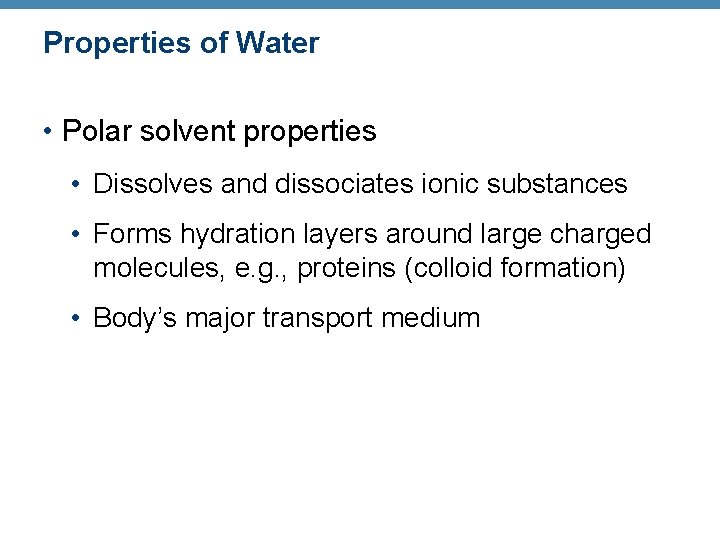
Properties of Water • Polar solvent properties • Dissolves and dissociates ionic substances • Forms hydration layers around large charged molecules, e. g. , proteins (colloid formation) • Body’s major transport medium

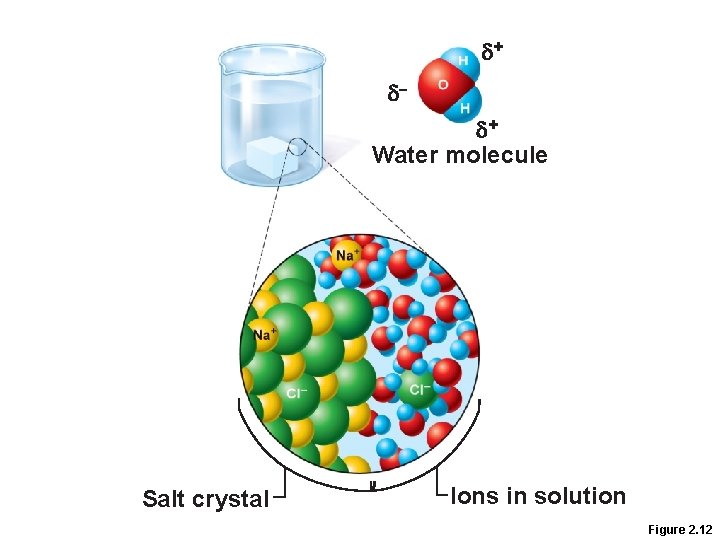
+ – + Water molecule Salt crystal Ions in solution Figure 2. 12

Properties of Water • Reactivity • A necessary part of hydrolysis and dehydration synthesis reactions • Cushioning • Protects certain organs from physical trauma, e. g. , cerebrospinal fluid

Salts • Ionic compounds that dissociate in water • Contain cations other than H+ and anions other than OH– • Ions (electrolytes) conduct electrical currents in solution • Ions play specialized roles in body functions (e. g. , sodium, potassium, calcium, and iron)

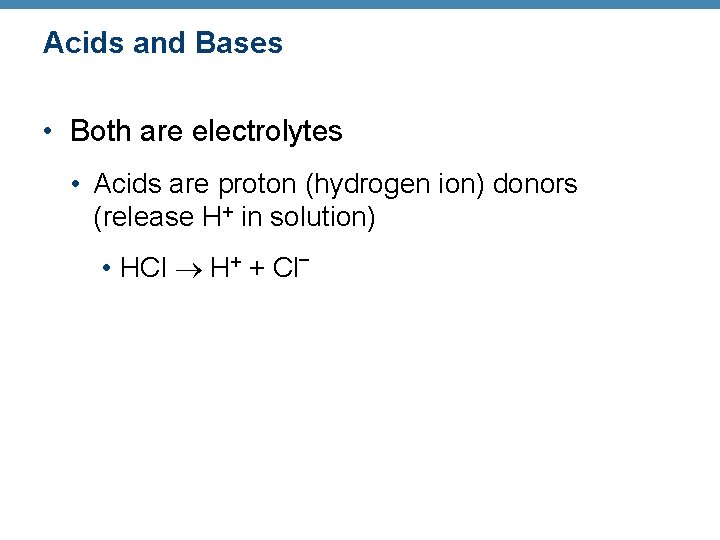
Acids and Bases • Both are electrolytes • Acids are proton (hydrogen ion) donors (release H+ in solution) • HCl H+ + Cl–

Acids and Bases • Bases are proton acceptors (take up H+ from solution) • Na. OH Na+ + OH– • OH– accepts an available proton (H+) • OH– + H+ H 2 O • Bicarbonate ion (HCO 3–) and ammonia (NH 3) are important bases in the body
![AcidBase Concentration Acid solutions contain H As H increases acidity increases Acid-Base Concentration • Acid solutions contain [H+] • As [H+] increases, acidity increases •](https://slidetodoc.com/presentation_image_h2/87619f5eccf050dfa6a50fa357333134/image-10.jpg)
Acid-Base Concentration • Acid solutions contain [H+] • As [H+] increases, acidity increases • Alkaline solutions contain bases (e. g. , OH–) • As [H+] decreases (or as [OH–] increases), alkalinity increases
![p H AcidBase Concentration p H the negative logarithm of H in p. H: Acid-Base Concentration • p. H = the negative logarithm of [H+] in](https://slidetodoc.com/presentation_image_h2/87619f5eccf050dfa6a50fa357333134/image-11.jpg)
p. H: Acid-Base Concentration • p. H = the negative logarithm of [H+] in moles per liter • Neutral solutions: • Pure water is p. H neutral (contains equal numbers of H+ and OH–) • p. H of pure water = p. H 7: [H+] = 10 – 7 M • All neutral solutions are p. H 7
![p H AcidBase Concentration Acidic solutions H p H Acidic p p. H: Acid-Base Concentration • Acidic solutions • [H+], p. H • Acidic p.](https://slidetodoc.com/presentation_image_h2/87619f5eccf050dfa6a50fa357333134/image-12.jpg)
p. H: Acid-Base Concentration • Acidic solutions • [H+], p. H • Acidic p. H: 0– 6. 99 • p. H scale is logarithmic: a p. H 5 solution has 10 times more H+ than a p. H 6 solution • Alkaline solutions • [H+], p. H • Alkaline (basic) p. H: 7. 01– 14
![Concentration molesliter Examples OH H p H 100 10 14 14 1 M Sodium Concentration (moles/liter) Examples [OH–] [H+] p. H 100 10– 14 14 1 M Sodium](https://slidetodoc.com/presentation_image_h2/87619f5eccf050dfa6a50fa357333134/image-13.jpg)
Concentration (moles/liter) Examples [OH–] [H+] p. H 100 10– 14 14 1 M Sodium hydroxide (p. H=14) 10– 13 13 Oven cleaner, lye (p. H=13. 5) 10– 2 10– 12 12 10– 3 10– 11 11 10– 4 10– 10 10 10– 5 10– 9 9 10– 6 10– 8 8 10– 7 7 Neutral 10– 8 10– 6 6 10– 9 10– 5 5 10– 10 10– 4 4 10– 11 10– 3 3 10– 12 10– 2 2 10– 13 10– 1 1 10– 14 100 0 Household ammonia (p. H=10. 5– 11. 5) Household bleach (p. H=9. 5) Egg white (p. H=8) Blood (p. H=7. 4) Milk (p. H=6. 3– 6. 6) Black coffee (p. H=5) Wine (p. H=2. 5– 3. 5) Lemon juice; gastric juice (p. H=2) 1 M Hydrochloric acid (p. H=0) Figure 2. 13

Acid-Base Homeostasis • p. H change interferes with cell function and may damage living tissue • Slight change in p. H can be fatal • p. H is regulated by kidneys, lungs, and buffers

Buffers • Mixture of compounds that resist p. H changes • Convert strong (completely dissociated) acids or bases into weak (slightly dissociated) ones • Carbonic acid-bicarbonate system

Organic Compounds • Contain carbon (except CO 2 and CO, which are inorganic) • Unique to living systems • Include carbohydrates, lipids, proteins, and nucleic acids

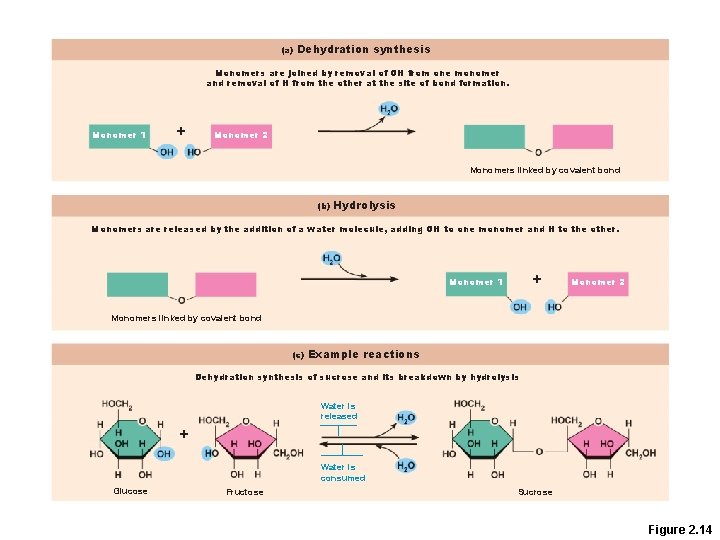
Organic Compounds • Many are polymers—chains of similar units (monomers or building blocks) • Synthesized by dehydration synthesis • Broken down by hydrolysis reactions

(a) Dehydration synthesis Monomers are joined by removal of OH from one monomer and removal of H from the other at the site of bond formation. Monomer 1 + Monomer 2 Monomers linked by covalent bond (b) Hydrolysis Monomers are released by the addition of a water molecule, adding OH to one monomer and H to the other. + Monomer 1 Monomer 2 Monomers linked by covalent bond (c) Example reactions Dehydration synthesis of sucrose and its breakdown by hydrolysis Water is released + Water is consumed Glucose Fructose Sucrose Figure 2. 14
![Carbohydrates Sugars and starches Contain C H and O CH 20n Carbohydrates • Sugars and starches • Contain C, H, and O [(CH 20)n] •](https://slidetodoc.com/presentation_image_h2/87619f5eccf050dfa6a50fa357333134/image-19.jpg)
Carbohydrates • Sugars and starches • Contain C, H, and O [(CH 20)n] • Three classes • Monosaccharides • Disaccharides • Polysaccharides

Carbohydrates • Functions • Major source of cellular fuel (e. g. , glucose) • Structural molecules (e. g. , ribose sugar in RNA)

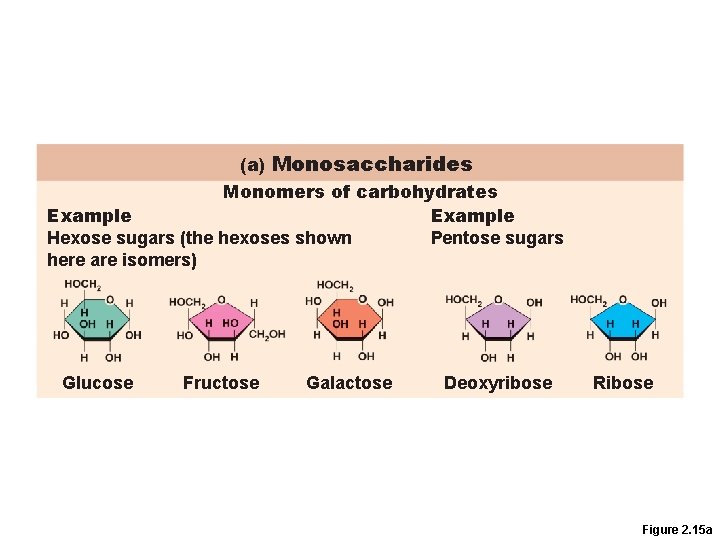
Monosaccharides • Simple sugars containing three to seven C atoms • (CH 20)n

(a) Monosaccharides Monomers of carbohydrates Example Hexose sugars (the hexoses shown Pentose sugars here are isomers) Glucose Fructose Galactose Deoxyribose Ribose Figure 2. 15 a

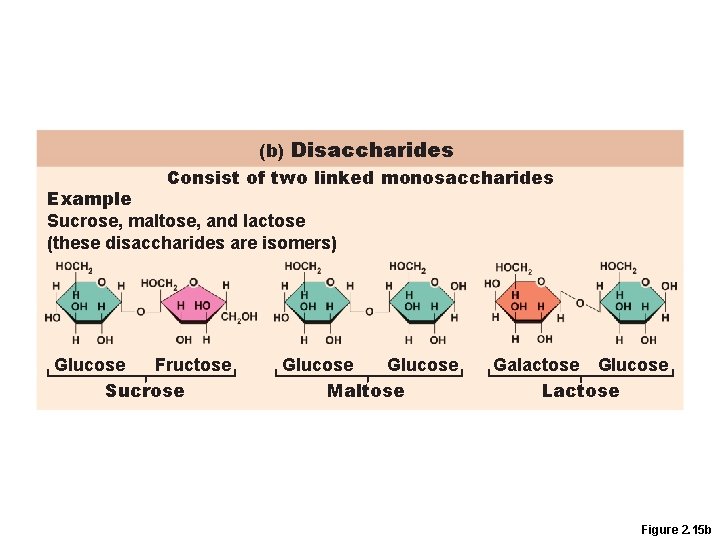
Disaccharides • Double sugars • Too large to pass through cell membranes

(b) Disaccharides Consist of two linked monosaccharides Example Sucrose, maltose, and lactose (these disaccharides are isomers) Glucose Fructose Sucrose Glucose Maltose Galactose Glucose Lactose Figure 2. 15 b

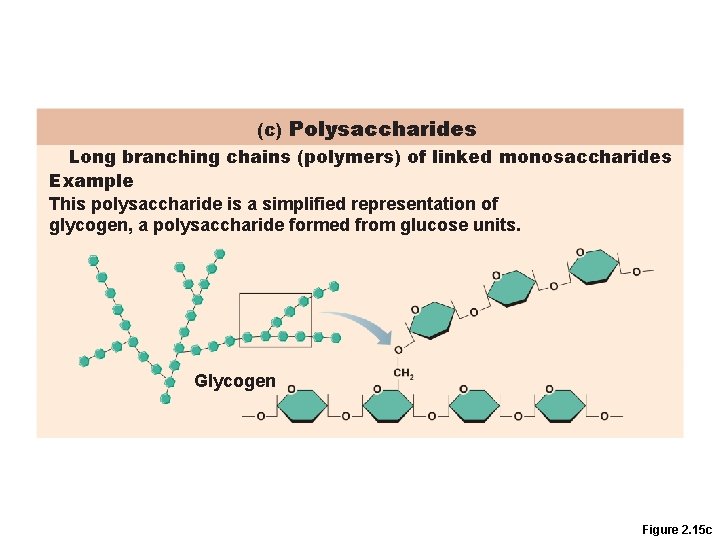
Polysaccharides • Polymers of simple sugars, e. g. , starch and glycogen • Not very soluble

(c) Polysaccharides Long branching chains (polymers) of linked monosaccharides Example This polysaccharide is a simplified representation of glycogen, a polysaccharide formed from glucose units. Glycogen Figure 2. 15 c
 Water and water and water water
Water and water and water water Ferric alum as a double salt
Ferric alum as a double salt Organic and inorganic compounds experiment
Organic and inorganic compounds experiment Organic chemistry introduction
Organic chemistry introduction Organic vs inorganic compounds
Organic vs inorganic compounds Organic vs inorganic compounds
Organic vs inorganic compounds Pharmaceutical inorganic chemistry introduction
Pharmaceutical inorganic chemistry introduction Organic versus inorganic compounds
Organic versus inorganic compounds Classes e subclasses
Classes e subclasses Pre ap classes vs regular classes
Pre ap classes vs regular classes Chapter 19 acids bases and salts worksheet answer key
Chapter 19 acids bases and salts worksheet answer key Chapter 19 acids bases and salts
Chapter 19 acids bases and salts Chapter 19 acids bases and salts
Chapter 19 acids bases and salts Acid bases and salts
Acid bases and salts Chapter 19 acids bases and salts
Chapter 19 acids bases and salts Hso4na
Hso4na 2282021
2282021 Examples of double displacement reaction
Examples of double displacement reaction Covalent and ionic bonds venn diagram
Covalent and ionic bonds venn diagram What is heat of neutralization
What is heat of neutralization Amine functional group
Amine functional group How to name esters
How to name esters In addition to moving horizontally ocean water moves
In addition to moving horizontally ocean water moves Bile salts name
Bile salts name White rush bath salts
White rush bath salts Properties of acids and bases
Properties of acids and bases Acid base properties of salts
Acid base properties of salts