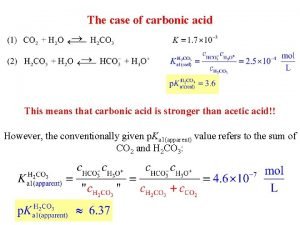
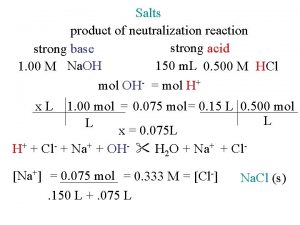
Salts product of neutralization reaction strong acid strong
- Slides: 7

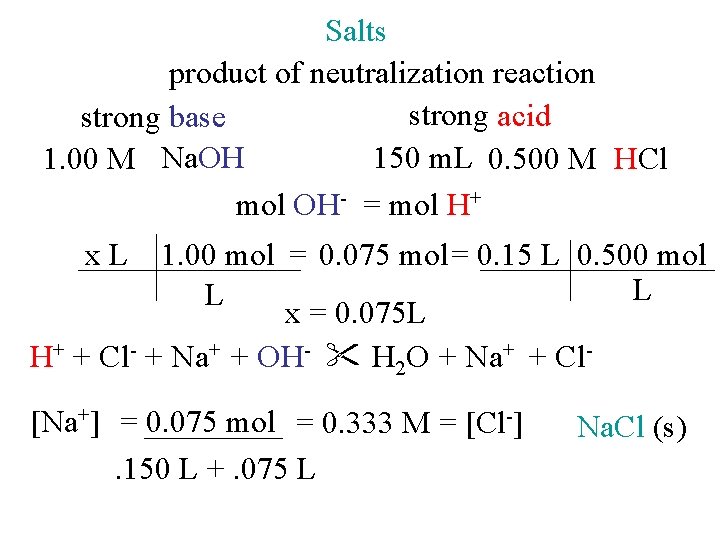
Salts product of neutralization reaction strong acid strong base 150 m. L 0. 500 M HCl 1. 00 M Na. OH mol OH- = mol H+ x. L 1. 00 mol = 0. 075 mol = 0. 15 L 0. 500 mol L L x = 0. 075 L H+ + Cl- + Na+ + OH- H 2 O + Na+ + Cl[Na+] = 0. 075 mol = 0. 333 M = [Cl-]. 150 L +. 075 L Na. Cl (s)

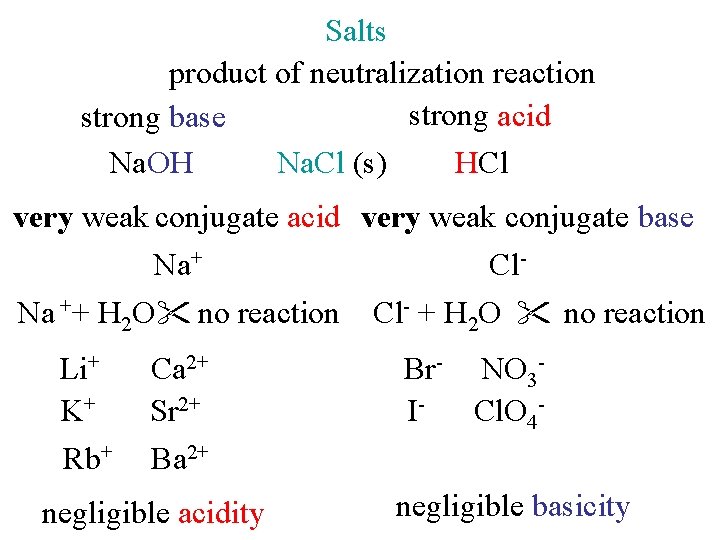
Salts product of neutralization reaction strong acid strong base Na. OH Na. Cl (s) HCl very weak conjugate acid very weak conjugate base Na+ Cl. Na ++ H 2 O no reaction Cl- + H 2 O no reaction Li+ K+ Rb+ Ca 2+ Sr 2+ Ba 2+ negligible acidity Br- NO 3 ICl. O 4 negligible basicity

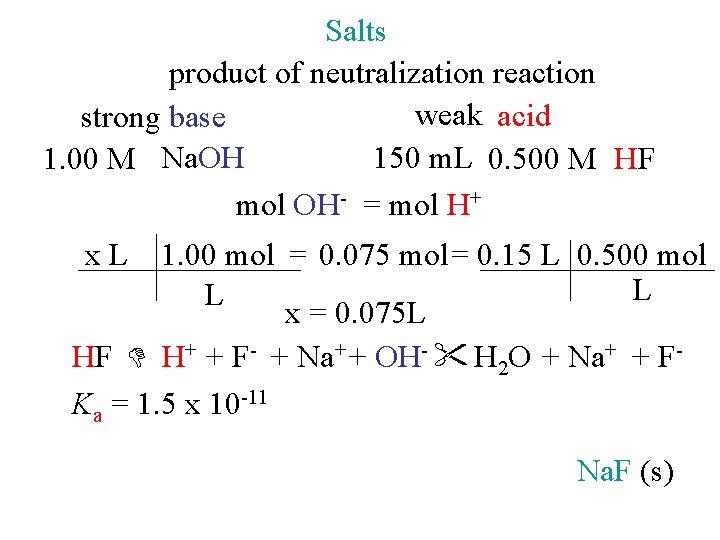
Salts product of neutralization reaction weak acid strong base 150 m. L 0. 500 M HF 1. 00 M Na. OH mol OH- = mol H+ x. L 1. 00 mol = 0. 075 mol = 0. 15 L 0. 500 mol L L x = 0. 075 L HF H+ + F- + Na+ + OH- H 2 O + Na+ + FKa = 1. 5 x 10 -11 Na. F (s)

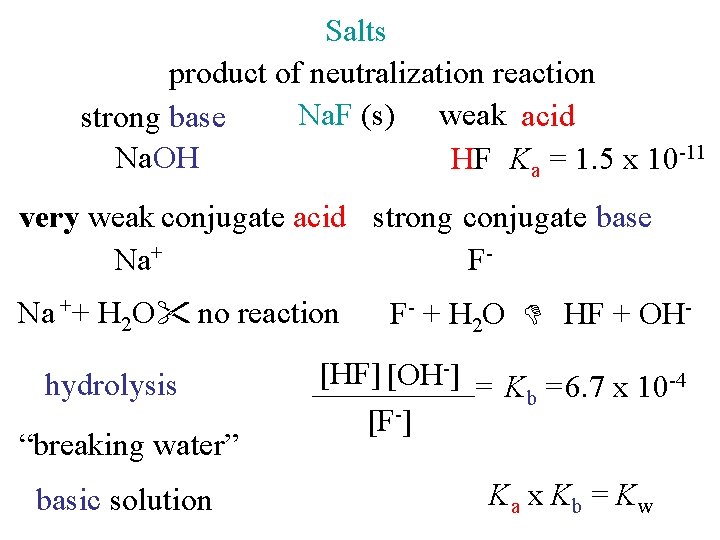
Salts product of neutralization reaction Na. F (s) weak acid strong base Na. OH HF Ka = 1. 5 x 10 -11 very weak conjugate acid strong conjugate base Na+ FNa ++ H 2 O no reaction hydrolysis “breaking water” basic solution F- + H 2 O HF + OH- [HF] [OH-] = K =6. 7 x 10 -4 b [F-] Ka x Kb = Kw

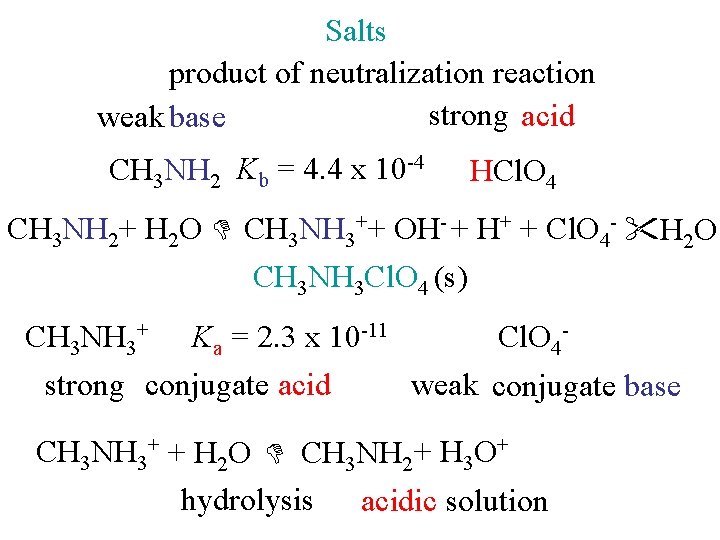
Salts product of neutralization reaction strong acid weak base CH 3 NH 2 Kb = 4. 4 x 10 -4 HCl. O 4 CH 3 NH 2+ H 2 O CH 3 NH 3++ OH- + H+ + Cl. O 4 - H 2 O CH 3 NH 3 Cl. O 4 (s) CH 3 NH 3+ Ka = 2. 3 x 10 -11 Cl. O 4 strong conjugate acid weak conjugate base CH 3 NH 3+ + H 2 O CH 3 NH 2 + H 3 O+ hydrolysis acidic solution

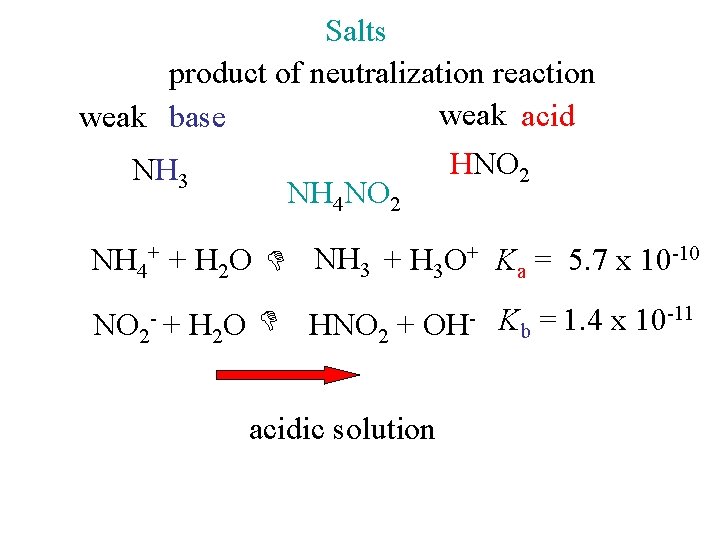
Salts product of neutralization reaction weak acid weak base HNO 2 NH 3 NH 4 NO 2 NH 4+ + H 2 O NH 3 + H 3 O+ Ka = 5. 7 x 10 -10 NO 2 - + H 2 O HNO 2 + OH- Kb = 1. 4 x 10 -11 acidic solution

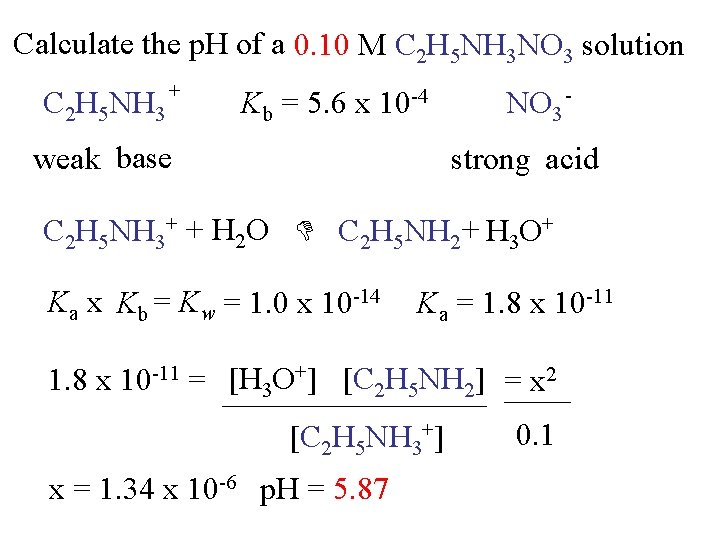
Calculate the p. H of a 0. 10 M C 2 H 5 NH 3 NO 3 solution C 2 H 5 NH 3 + Kb = 5. 6 x 10 -4 weak base NO 3 strong acid C 2 H 5 NH 3+ + H 2 O C 2 H 5 NH 2 + H 3 O+ Ka x Kb = Kw = 1. 0 x 10 -14 Ka = 1. 8 x 10 -11 = [H 3 O+] [C 2 H 5 NH 2] = x 2 [C 2 H 5 NH 3+] x = 1. 34 x 10 -6 p. H = 5. 87 0. 1