Acid Neutralization reactor Module 4 Acid neutralization reactor











- Slides: 41

Acid Neutralization reactor Module 4: Acid neutralization reactor Lecture 3: Acid - base chemistry, p. H and the equations for a CSTR for a reacting system Mark J. Mc. Cready Chemical Engineering Introduction to Engineering Systems Module 4 - Acid Neutralization Reactor Copyright © 2001, University of Notre Dame

Outline for today n Review: u Feedback control of a chemical reactor F n Proportional and integral control p. H and p. H measurement We will monitor progress of reaction this way u p. H is a design criterion for outlet stream u n Weak acid chemistry and buffer solutions Acetic acid dissociation u Neutralization reaction u n Design equations for the chemical reactor Introduction to Engineering Systems Module 4 - Acid Neutralization Reactor Copyright © 2001, University of Notre Dame

Control of a chemical reactor n The next few slides are a photo gallery showing the expected behavior of a chemical reactor with u Proportional control F u Integral control F u base flowrate is proportional to error in p. H flow rate of base is related to accumulated error in p. H Proportional and integral control together. Introduction to Engineering Systems Module 4 - Acid Neutralization Reactor Copyright © 2001, University of Notre Dame

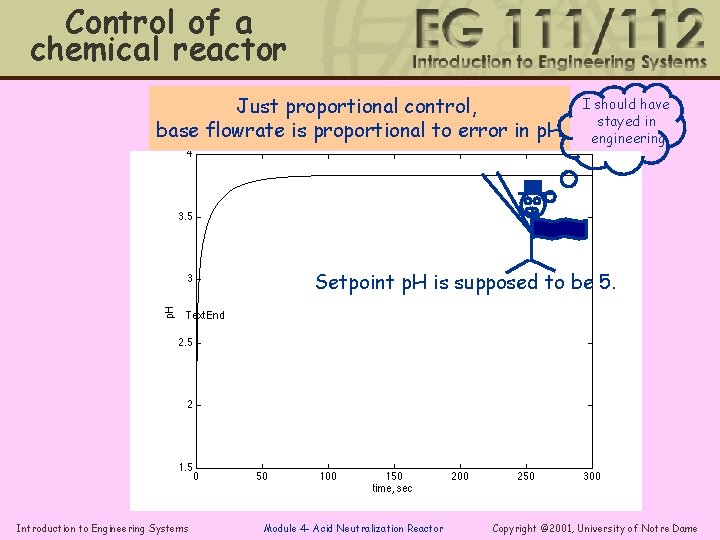
Control of a chemical reactor Just proportional control, base flowrate is proportional to error in p. H I should have stayed in engineering Setpoint p. H is supposed to be 5. Introduction to Engineering Systems Module 4 - Acid Neutralization Reactor Copyright © 2001, University of Notre Dame

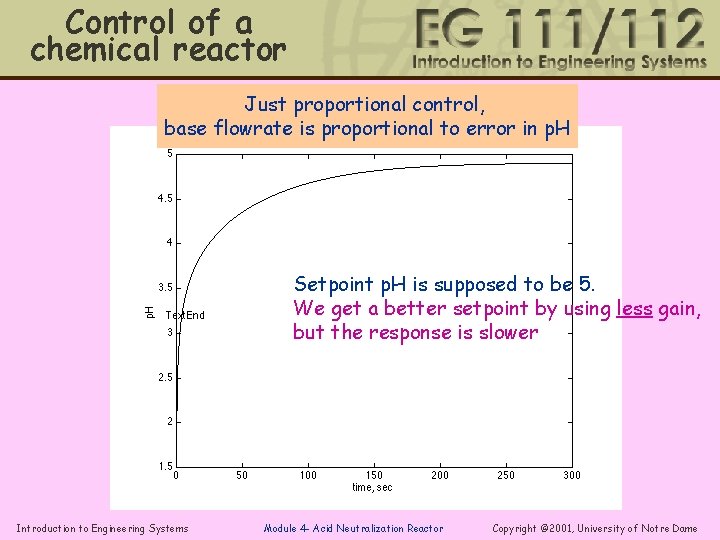
Control of a chemical reactor Just proportional control, base flowrate is proportional to error in p. H Setpoint p. H is supposed to be 5. We get a better setpoint by using less gain, but the response is slower Introduction to Engineering Systems Module 4 - Acid Neutralization Reactor Copyright © 2001, University of Notre Dame

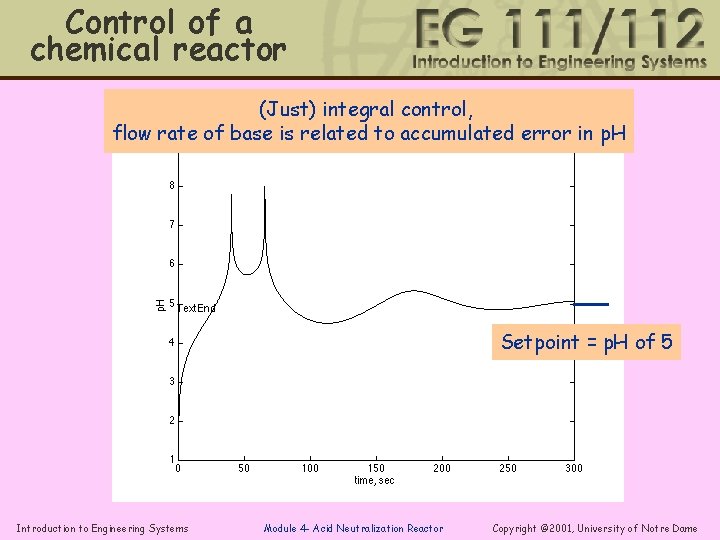
Control of a chemical reactor (Just) integral control, flow rate of base is related to accumulated error in p. H Setpoint = p. H of 5 Introduction to Engineering Systems Module 4 - Acid Neutralization Reactor Copyright © 2001, University of Notre Dame

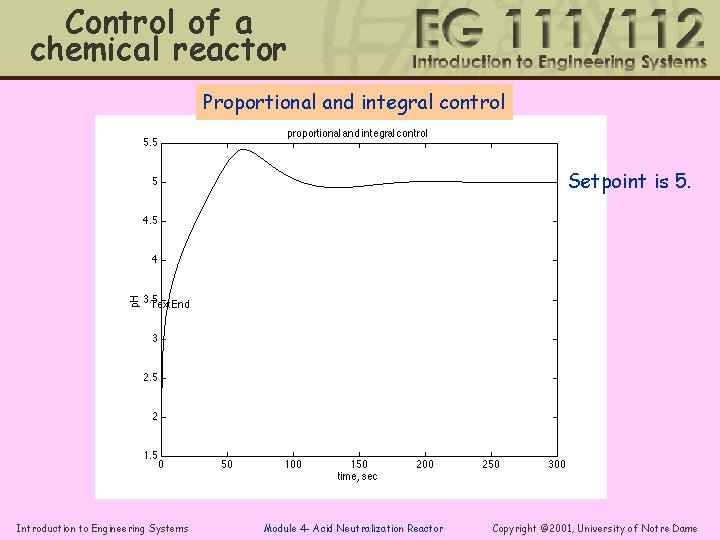
Control of a chemical reactor Proportional and integral control Setpoint is 5. Introduction to Engineering Systems Module 4 - Acid Neutralization Reactor Copyright © 2001, University of Notre Dame

Control of a chemical reactor Proportional and integral control Setpoint is 5. Introduction to Engineering Systems Module 4 - Acid Neutralization Reactor Copyright © 2001, University of Notre Dame

Dissociation of water n n You may recall that any aqueous solution (or “pure” water) contains hydronium and hydroxide ions because of the dissociation of water. This equilibrium relation is: If the solution is neutral, the concentrations of the two ions are equal. If not, solution is either basic (more OH-) or acidic (more H 3 O + ) Introduction to Engineering Systems Module 4 - Acid Neutralization Reactor Copyright © 2001, University of Notre Dame

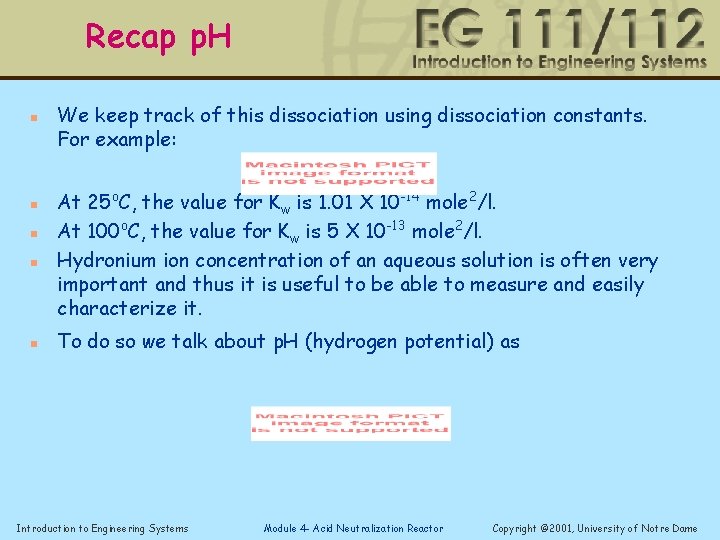
Dissociation of water n n n We keep track of this dissociation using dissociation constants. For example: At 25 o. C, the value for Kw is 1. 01 X 10 -14 mole 2/l. At 100 o. C, the value for Kw is 5 X 10 -13 mole 2/l. Hydronium ion concentration of an aqueous solution is often very important and thus it is useful to be able to measure and easily characterize it. To do so we talk about p. H (hydrogen potential) as Introduction to Engineering Systems Module 4 - Acid Neutralization Reactor Copyright © 2001, University of Notre Dame

How can we measure p. H ? n n Modern electrodes and electronics allow measurement of p. H (except at extreme values) quite easily. You could make mistakes, but these should be pretty small in this experiment Introduction to Engineering Systems Module 4 - Acid Neutralization Reactor Copyright © 2001, University of Notre Dame

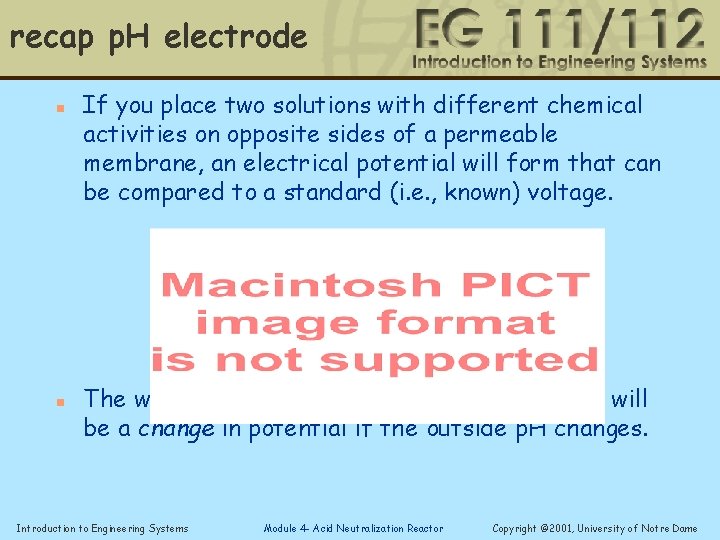
Working principle of a p. H electrode n n If you place two solutions with different chemical activities on opposite sides of a permeable membrane, an electrical potential will form that can be compared to a standard (i. e. , known) voltage. The way this is used for measurement is there will be a change in potential if the outside p. H changes. Introduction to Engineering Systems Module 4 - Acid Neutralization Reactor Copyright © 2001, University of Notre Dame

Workings of an electrode n n The key to a p. H electrode is a thin membrane of special glass. Glass is sensitive to H 3 O+ ions, but not other singly charged ions. p. H is then determined from the potential between the p. H electrode and a standard reference electrode Introduction to Engineering Systems Module 4 - Acid Neutralization Reactor Copyright © 2001, University of Notre Dame

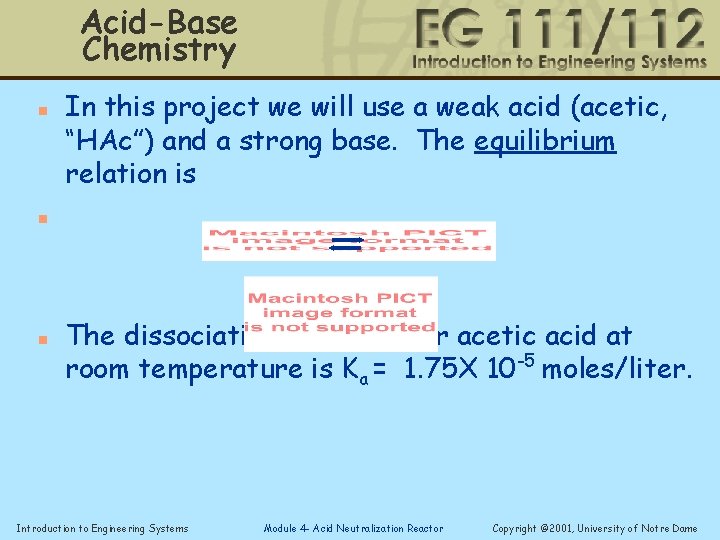
Acid-Base Chemistry n In this project we will use a weak acid (acetic, “HAc”) and a strong base. The equilibrium relation is n n The dissociation constant for acetic acid at room temperature is Ka = 1. 75 X 10 -5 moles/liter. Introduction to Engineering Systems Module 4 - Acid Neutralization Reactor Copyright © 2001, University of Notre Dame

p. H of acid solution n n If we want the p. H of our acetic acid solution we start with the equilibrium relation We want [H 3 O+] and we know Ka. Thus we need two more equations to get [Ac-] and [HAc] We have available the mass balance for total acetate. + We can also be pretty sure that all of the [H O ] comes fromup) the 3 (c. HAc is the concentration you made acetic acid (it is not from the little that would be present in pure water) so that Introduction to Engineering Systems (each Ac- must have an H 3 O+) Module 4 - Acid Neutralization Reactor Copyright © 2001, University of Notre Dame

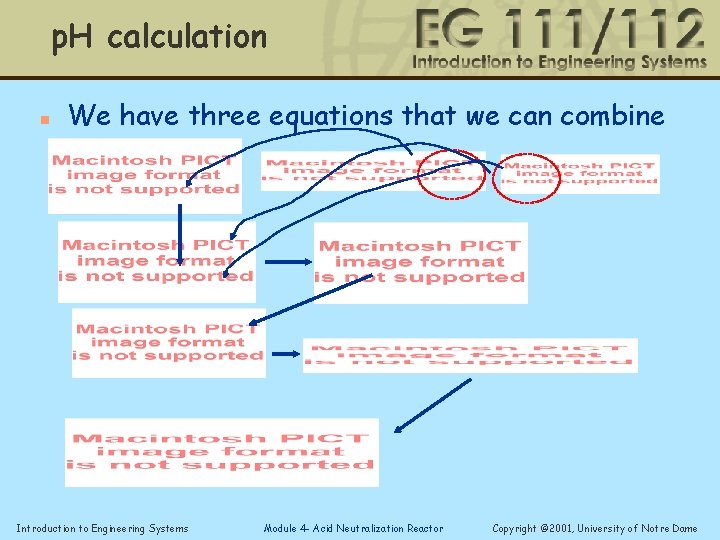
p. H calculation n We have three equations that we can combine Introduction to Engineering Systems Module 4 - Acid Neutralization Reactor Copyright © 2001, University of Notre Dame

p. H calculation n We have three equations that we can combine Introduction to Engineering Systems Module 4 - Acid Neutralization Reactor Copyright © 2001, University of Notre Dame

p. H calculation n We have three equations that we can combine Introduction to Engineering Systems Module 4 - Acid Neutralization Reactor Copyright © 2001, University of Notre Dame

p. H numbers n n We can put in numbers Ka = 1. 75 X 10 -5 moles/liter, c. HAc = 1 mole/liter Introduction to Engineering Systems Module 4 - Acid Neutralization Reactor Copyright © 2001, University of Notre Dame

An Aside n n n So, you don’t like lectures !!! If you have come to all of them, so far you have been to about 26 lectures, there are 6 remaining. You would have been to 81% of the total classes so far. . Introduction to Engineering Systems Module 4 - Acid Neutralization Reactor Copyright © 2001, University of Notre Dame

Buffer solutions n n Once you begin to titrate the acetic acid with sodium hydroxide, you begin producing sodium acetate and water. The presence of both a weak acid and its “conjugate base” (i. e. , sodium acetate) constitutes a “buffer solution”. It is called this because it resists changes in p. H caused by the addition of a strong base or pure water. Introduction to Engineering Systems Module 4 - Acid Neutralization Reactor Copyright © 2001, University of Notre Dame

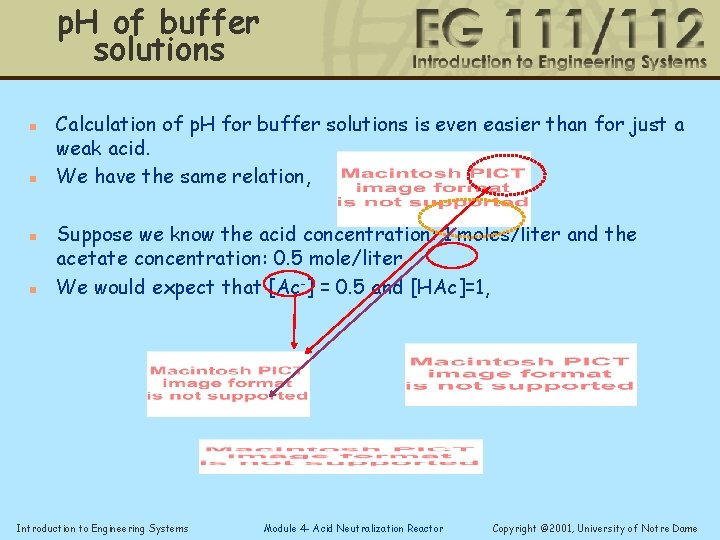
p. H of buffer solutions n n Calculation of p. H for buffer solutions is even easier than for just a weak acid. We have the same relation, Suppose we know the acid concentration: 1 moles/liter and the acetate concentration: 0. 5 mole/liter We would expect that [Ac-] = 0. 5 and [HAc]=1, Introduction to Engineering Systems Module 4 - Acid Neutralization Reactor Copyright © 2001, University of Notre Dame

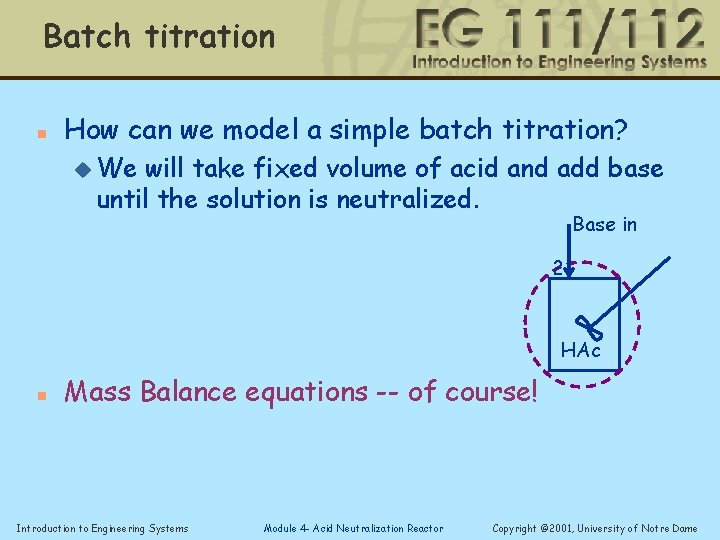
Batch titration n How can we model a simple batch titration? u We will take fixed volume of acid and add base until the solution is neutralized. Base in 2 HAc n Mass Balance equations -- of course! Introduction to Engineering Systems Module 4 - Acid Neutralization Reactor Copyright © 2001, University of Notre Dame

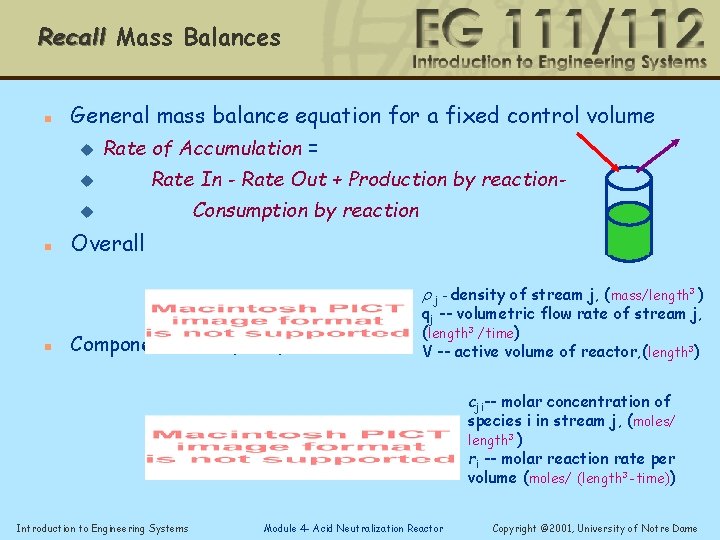
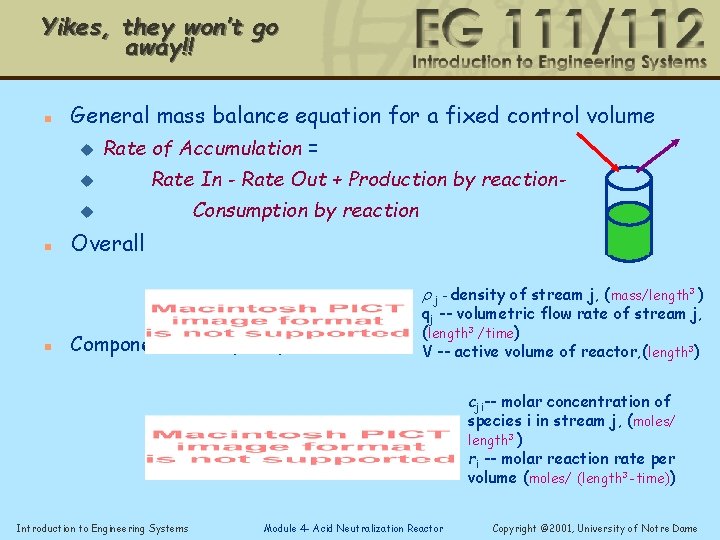
Recall Mass Balances n General mass balance equation for a fixed control volume u Rate of Accumulation = u Rate In - Rate Out + Production by reaction- u n Consumption by reaction Overall r j - density of stream j, (mass/length 3 ) n Component mass (mole) balance qj -- volumetric flow rate of stream j, (length 3 /time) V -- active volume of reactor, (length 3) cji-- molar concentration of species i in stream j, (moles/ length 3 ) ri -- molar reaction rate per volume (moles/ (length 3 -time)) Introduction to Engineering Systems Module 4 - Acid Neutralization Reactor Copyright © 2001, University of Notre Dame

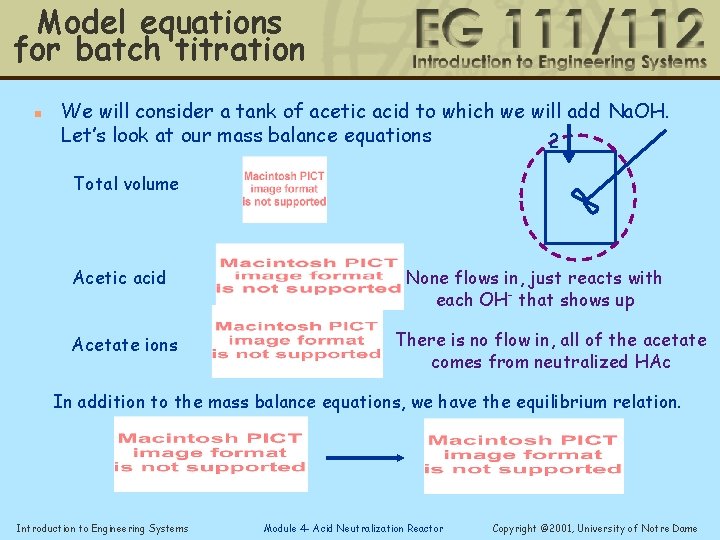
Model equations for batch titration n We will consider a tank of acetic acid to which we will add Na. OH. Let’s look at our mass balance equations 2 Total volume Acetic acid Acetate ions None flows in, just reacts with each OH- that shows up There is no flow in, all of the acetate comes from neutralized HAc In addition to the mass balance equations, we have the equilibrium relation. Introduction to Engineering Systems Module 4 - Acid Neutralization Reactor Copyright © 2001, University of Notre Dame

batch titration (cont. ) n n The problem is, what are the reaction terms? Let’s do a mass balance for OH-. We can see that it comes in by our inlet stream and it will all react away until all of the acid is used up. Further, the concentration of OH- in the reactor will be almost 0 (solution is acidic) until the acid is used up. For every mole of OH- used up we must react one mole of H 3 O+. Thus we use up one mole of HAc. We likewise make a mole of Acetate, Introduction to Engineering Systems Module 4 - Acid Neutralization Reactor Copyright © 2001, University of Notre Dame

batch titration (cont. ) n n The problem is, what are the reaction terms? Let’s do a mass balance for OH-. We can see that it comes in by our inlet stream and it will all react away until all of the acid is used up. Further, the concentration of OH- in the reactor will be almost 0 (solution is acidic) until the acid is used up. For every mole of OH- used up we must react one mole of H 3 O+. Thus we use up one mole of HAc. We likewise make a mole of Acetate, Introduction to Engineering Systems Module 4 - Acid Neutralization Reactor Copyright © 2001, University of Notre Dame

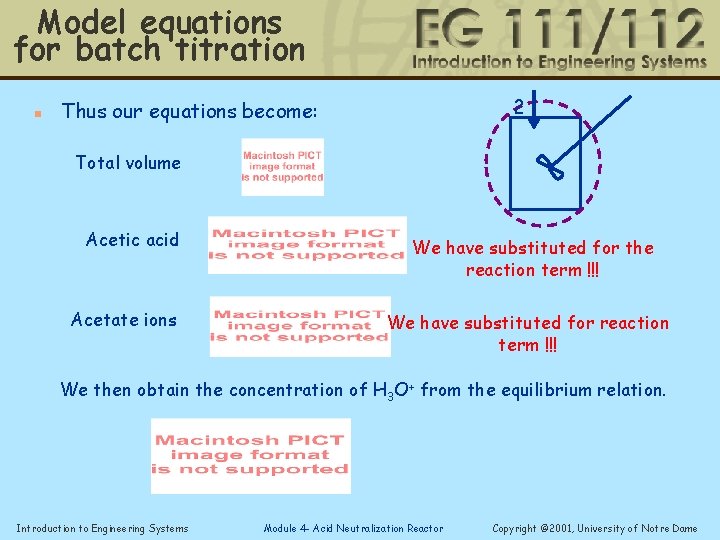
Model equations for batch titration n 2 Thus our equations become: Total volume Acetic acid Acetate ions We have substituted for the reaction term !!! We have substituted for reaction term !!! We then obtain the concentration of H 3 O+ from the equilibrium relation. Introduction to Engineering Systems Module 4 - Acid Neutralization Reactor Copyright © 2001, University of Notre Dame

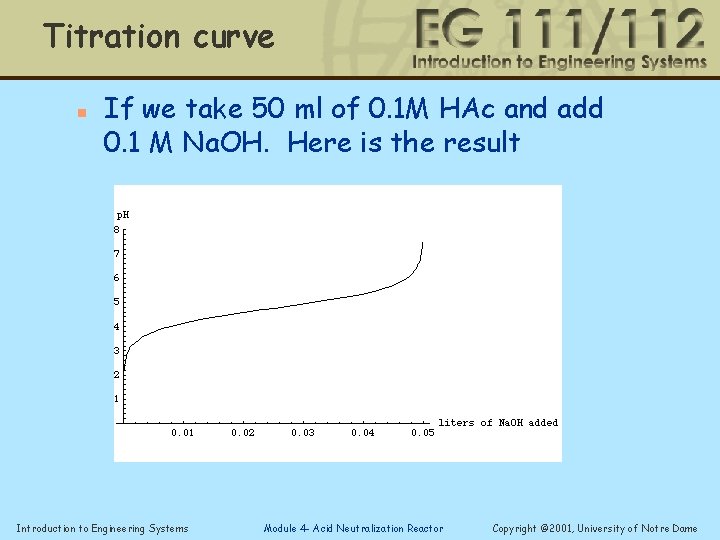
Titration curve n If we take 50 ml of 0. 1 M HAc and add 0. 1 M Na. OH. Here is the result Introduction to Engineering Systems Module 4 - Acid Neutralization Reactor Copyright © 2001, University of Notre Dame

Yikes, they won’t go away!! n General mass balance equation for a fixed control volume u Rate of Accumulation = u Rate In - Rate Out + Production by reaction- u n Consumption by reaction Overall r j - density of stream j, (mass/length 3 ) n Component mass (mole) balance qj -- volumetric flow rate of stream j, (length 3 /time) V -- active volume of reactor, (length 3) cji-- molar concentration of species i in stream j, (moles/ length 3 ) ri -- molar reaction rate per volume (moles/ (length 3 -time)) Introduction to Engineering Systems Module 4 - Acid Neutralization Reactor Copyright © 2001, University of Notre Dame

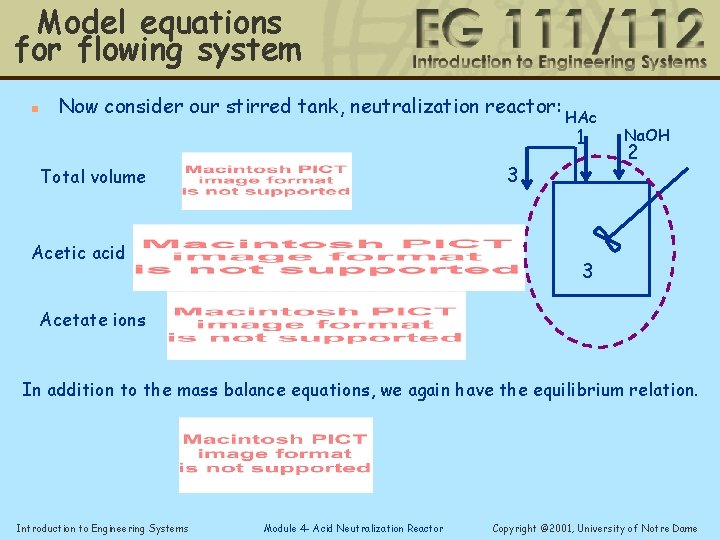
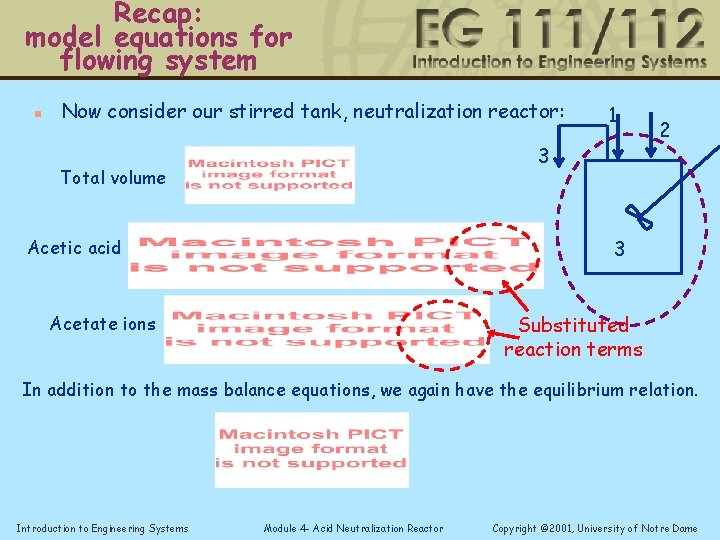
Model equations for flowing system n Now consider our stirred tank, neutralization reactor: HAc 1 3 Total volume Acetic acid Na. OH 2 3 Acetate ions In addition to the mass balance equations, we again have the equilibrium relation. Introduction to Engineering Systems Module 4 - Acid Neutralization Reactor Copyright © 2001, University of Notre Dame

Flowing system model (cont. ) n n Again, we don’t yet know the reaction terms. What are they? Again we do a mass balance for OH-. We can see that it comes in by our inlet stream and it will all react away until all of the acid is used up. Further, the concentration of OH- in the reactor will be almost 0 (solution is acidic) until the acid is used up. For every mole of OH- used up we must react one mole of H 3 O+. Thus we use up one mole of HAc. So we again get: We likewise make a mole of Acetate, Introduction to Engineering Systems Module 4 - Acid Neutralization Reactor Copyright © 2001, University of Notre Dame

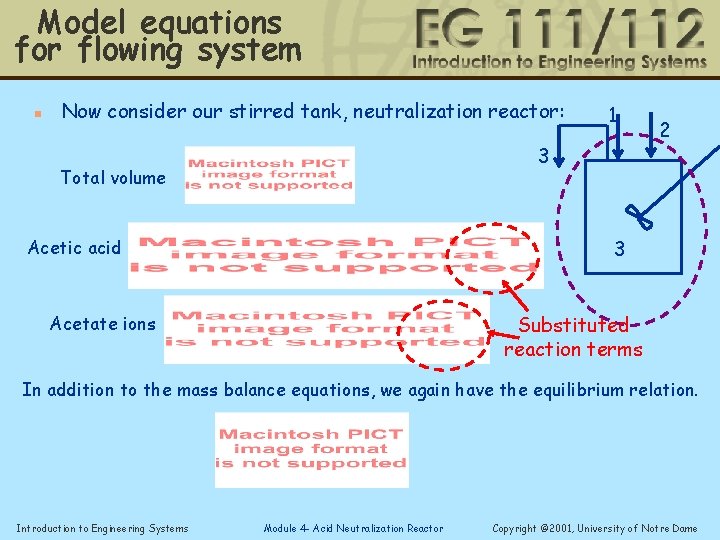
Model equations for flowing system n Now consider our stirred tank, neutralization reactor: 1 3 Total volume 2 3 Acetic acid Substituted reaction terms Acetate ions In addition to the mass balance equations, we again have the equilibrium relation. Introduction to Engineering Systems Module 4 - Acid Neutralization Reactor Copyright © 2001, University of Notre Dame

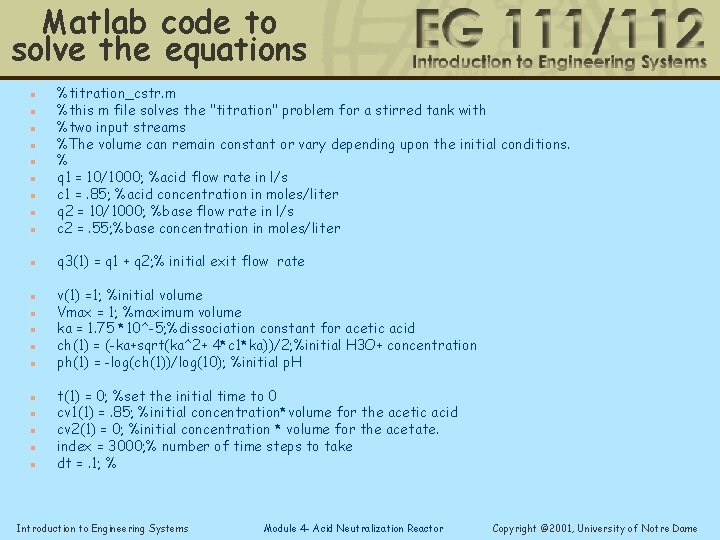
Matlab code to solve the equations n %titration_cstr. m %this m file solves the "titration" problem for a stirred tank with %two input streams %The volume can remain constant or vary depending upon the initial conditions. % q 1 = 10/1000; %acid flow rate in l/s c 1 =. 85; %acid concentration in moles/liter q 2 = 10/1000; %base flow rate in l/s c 2 =. 55; %base concentration in moles/liter n q 3(1) = q 1 + q 2; % initial exit flow rate n n n n n v(1) =1; %initial volume Vmax = 1; %maximum volume ka = 1. 75 *10^-5; %dissociation constant for acetic acid ch(1) = (-ka+sqrt(ka^2+ 4*c 1*ka))/2; %initial H 3 O+ concentration ph(1) = -log(ch(1))/log(10); %initial p. H t(1) = 0; %set the initial time to 0 cv 1(1) =. 85; %initial concentration*volume for the acetic acid cv 2(1) = 0; %initial concentration * volume for the acetate. index = 3000; % number of time steps to take dt =. 1; % Introduction to Engineering Systems Module 4 - Acid Neutralization Reactor Copyright © 2001, University of Notre Dame

Matlab code to solve these equations (pg 2) n n n n n % here is the loop for i=1: index t(i + 1) = t(i) + dt; %advance the time counter v(i + 1) = v(i) + (q 1 + q 2 - q 3(i))*dt; %get a new volume with Euler int. if v(i + 1) < Vmax %check to see if overflowing q 3(i + 1) = 0; %if not, don't change flow rate else q 3(i + 1) = q 1 + q 2 ; %if so, set outlet flow equal to inflow end %find the new V*c 1 using Euler integration cv 1(i + 1) = cv 1(i) + (q 1*c 1 - q 2*c 2 - q 3(i)*cv 1(i)/v(i))*dt; %find the new V*c 2 using Euler integration cv 2(i + 1) = cv 2(i) + ( q 2*c 2 - q 3(i)*cv 2(i)/v(i))*dt; ch(i + 1) = ka*cv 1(i + 1) /cv 2(i + 1) ; %calculate the new H 3 O+ concentration ph(i + 1) = -log(ch(i + 1))/log(10); %calculate the p. H end Introduction to Engineering Systems Module 4 - Acid Neutralization Reactor Copyright © 2001, University of Notre Dame

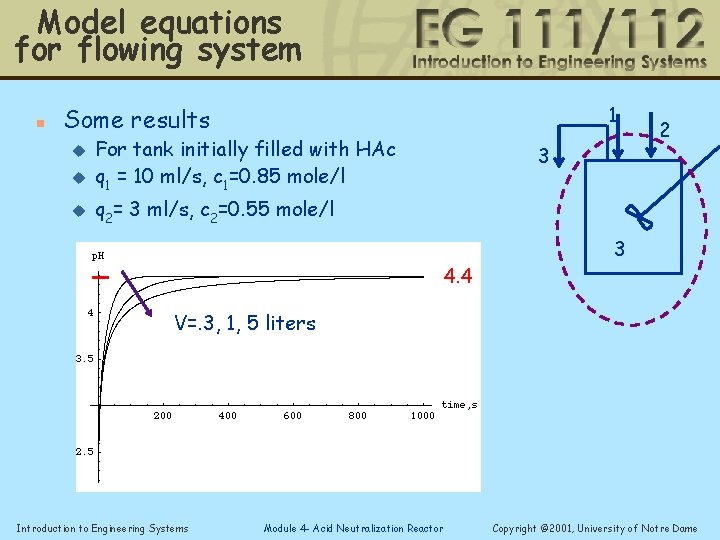
Model equations for flowing system n 1 Some results u For tank initially filled with HAc q 1 = 10 ml/s, c 1=0. 85 mole/l u q 2= 3 ml/s, c 2=0. 55 mole/l u 3 4. 4 2 3 V=. 3, 1, 5 liters Introduction to Engineering Systems Module 4 - Acid Neutralization Reactor Copyright © 2001, University of Notre Dame

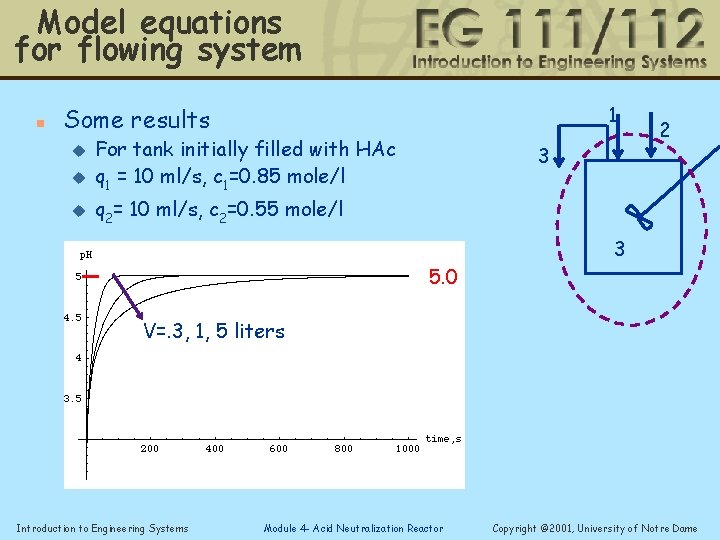
Model equations for flowing system n 1 Some results u For tank initially filled with HAc q 1 = 10 ml/s, c 1=0. 85 mole/l u q 2= 10 ml/s, c 2=0. 55 mole/l u 3 5. 0 2 3 V=. 3, 1, 5 liters Introduction to Engineering Systems Module 4 - Acid Neutralization Reactor Copyright © 2001, University of Notre Dame

Recap p. H n n n We keep track of this dissociation using dissociation constants. For example: At 25 o. C, the value for Kw is 1. 01 X 10 -14 mole 2/l. At 100 o. C, the value for Kw is 5 X 10 -13 mole 2/l. Hydronium ion concentration of an aqueous solution is often very important and thus it is useful to be able to measure and easily characterize it. To do so we talk about p. H (hydrogen potential) as Introduction to Engineering Systems Module 4 - Acid Neutralization Reactor Copyright © 2001, University of Notre Dame

recap p. H electrode n n If you place two solutions with different chemical activities on opposite sides of a permeable membrane, an electrical potential will form that can be compared to a standard (i. e. , known) voltage. The way this is used for measurement is there will be a change in potential if the outside p. H changes. Introduction to Engineering Systems Module 4 - Acid Neutralization Reactor Copyright © 2001, University of Notre Dame

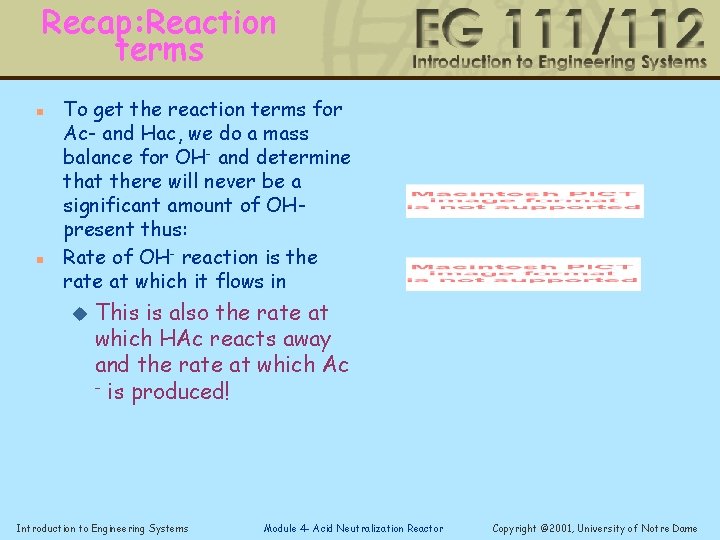
Recap: Reaction terms n n To get the reaction terms for Ac- and Hac, we do a mass balance for OH- and determine that there will never be a significant amount of OHpresent thus: Rate of OH- reaction is the rate at which it flows in u This is also the rate at which HAc reacts away and the rate at which Ac - is produced! Introduction to Engineering Systems Module 4 - Acid Neutralization Reactor Copyright © 2001, University of Notre Dame

Recap: model equations for flowing system n Now consider our stirred tank, neutralization reactor: 1 3 Total volume 2 3 Acetic acid Substituted reaction terms Acetate ions In addition to the mass balance equations, we again have the equilibrium relation. Introduction to Engineering Systems Module 4 - Acid Neutralization Reactor Copyright © 2001, University of Notre Dame