Acids Bases and Salts BASES ACIDS SALTS Acids
Acids, Bases and Salts BASES ACIDS SALTS

Acids ACIDS The word acids comes from one of its characteristic properties, its taste. ACIDS = “acidus” (latin word) meaning sour.

Acids ¡ ¡ An acid is a substance that releases H+ ions in an aqueous solution An aqueous solution is a solution that contains water. HCl H+ + Cl-
Bases are substances that are very slippery to touch and have a strong bitter taste. BASES
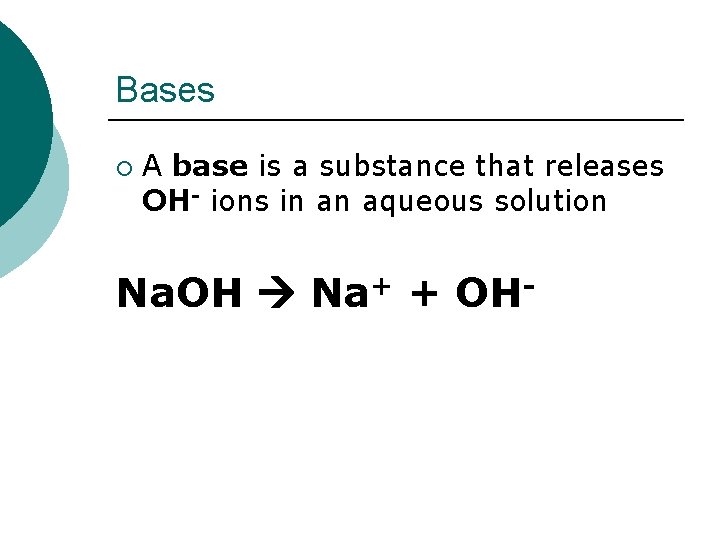
Bases ¡ A base is a substance that releases OH- ions in an aqueous solution Na. OH Na+ + OH-

Salts SALTS Salts are formed by the bonding of a metal with a non-metal. They are often used to change the physical properties of various solutions by lowering the freezing points or raising the boiling points

Salts ¡ Salts dissolve in water to produce ions (not including H+ or OH-) Na. Cl Na+ + Cl-
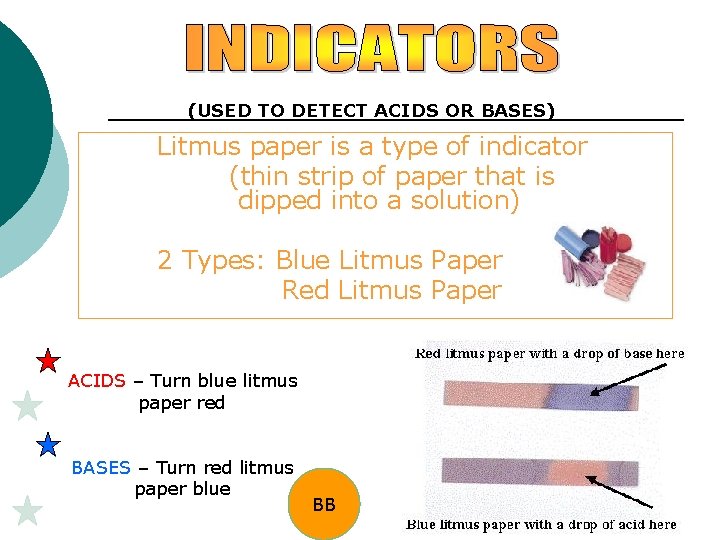
(USED TO DETECT ACIDS OR BASES) Litmus paper is a type of indicator (thin strip of paper that is dipped into a solution) 2 Types: Blue Litmus Paper Red Litmus Paper ACIDS – Turn blue litmus paper red BASES – Turn red litmus paper blue BB
Indicators SALTS – most salts are neutral
Indicators An indicator is a chemical used to tell if a liquid is an acid, base or neutral ¡ Phenolphthalein is another type of indicator ¡ Bases turn clear phenolphthalein pink ¡
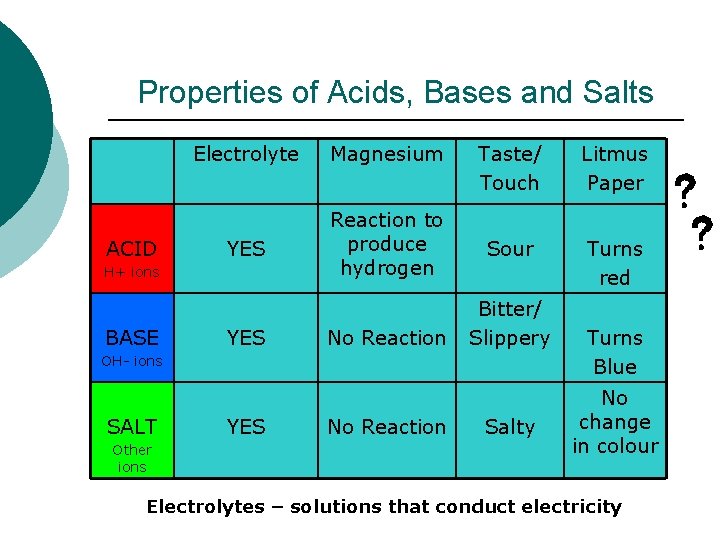
Properties of Acids, Bases and Salts ACID Electrolyte Magnesium YES Reaction to produce hydrogen H+ ions BASE YES No Reaction Taste/ Touch Litmus Paper Sour Turns red Bitter/ Slippery OH- ions SALT Other ions YES No Reaction Salty Turns Blue No change in colour Electrolytes – solutions that conduct electricity
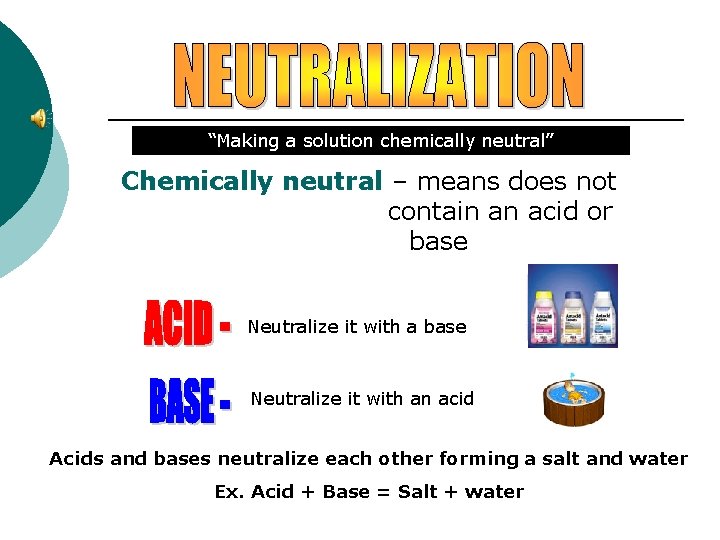
“Making a solution chemically neutral” Chemically neutral – means does not contain an acid or base Neutralize it with an acid Acids and bases neutralize each other forming a salt and water Ex. Acid + Base = Salt + water
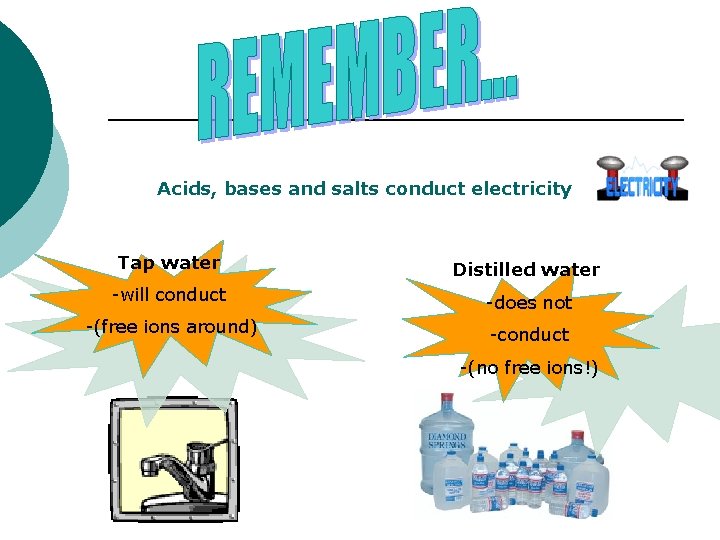
Acids, bases and salts conduct electricity Tap water Distilled water -will conduct -does not -(free ions around) -conduct -(no free ions!)
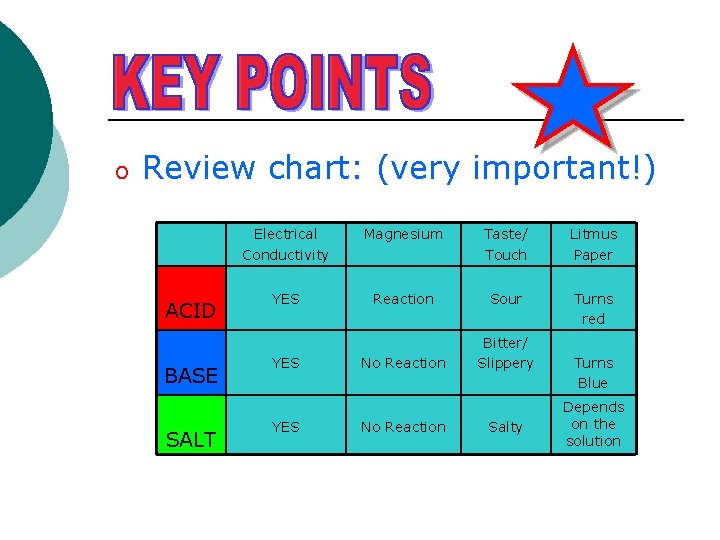
o Review chart: (very important!) ACID BASE SALT Electrical Conductivity Magnesium Taste/ Touch Litmus Paper YES Reaction Sour Turns red No Reaction Bitter/ Slippery YES No Reaction Salty Turns Blue Depends on the solution

Worksheet # 1 ¡ Study guide, Module 3, page 2 ¡
- Slides: 15