5 2 SALTS 1 What are salts Salts



















- Slides: 30

5. 2 SALTS 1

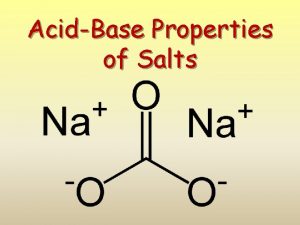
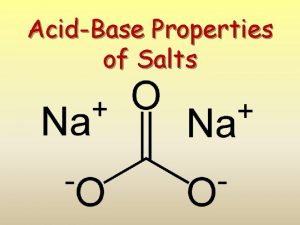
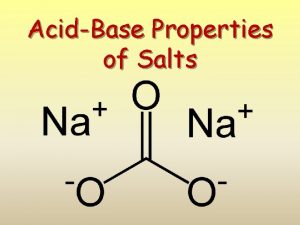
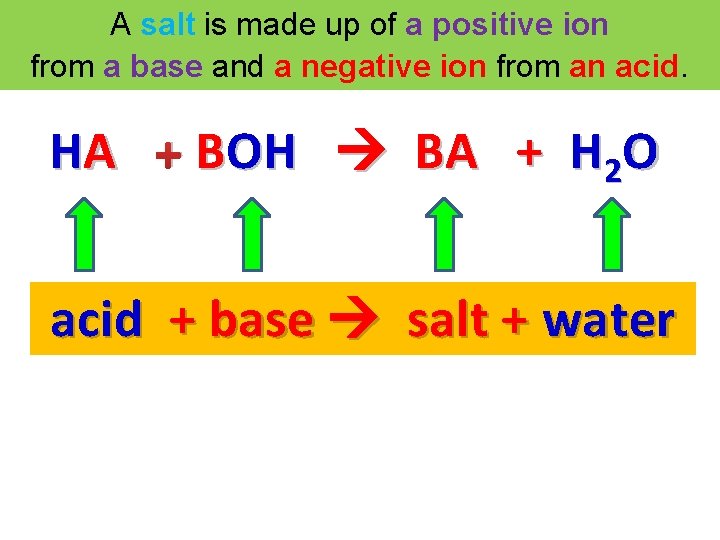
What are salts? Salts are a class of ionic compounds that can be produced when an acid and a base react H A + BOH OH BA + H 2 O acid + base salt + water

A salt is made up of a positive ion from a base and a negative ion from an acid. HA + BOH BA + H 2 O acid + base salt + water HCl + KOH KCl + H 2 O

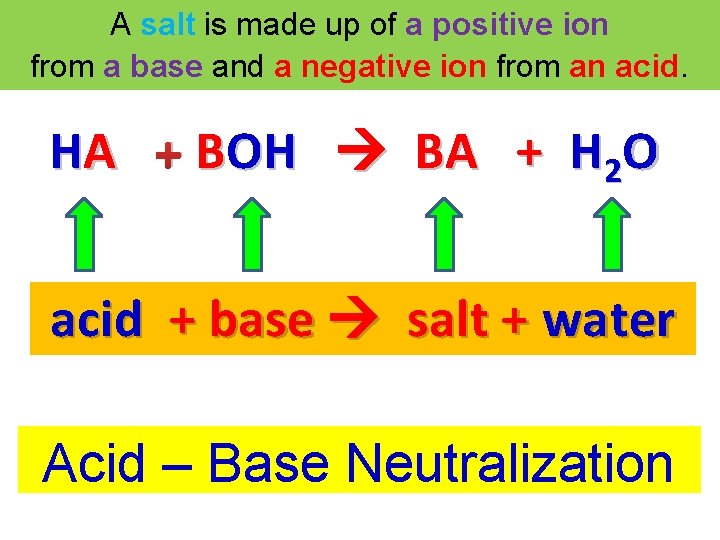
A salt is made up of a positive ion from a base and a negative ion from an acid. HA + BOH BA + H 2 O acid + base salt + water Acid – Base Neutralization

Acid – Base Neutralization HA + BOH BA + HOH HA + BOH BA + H 2 O

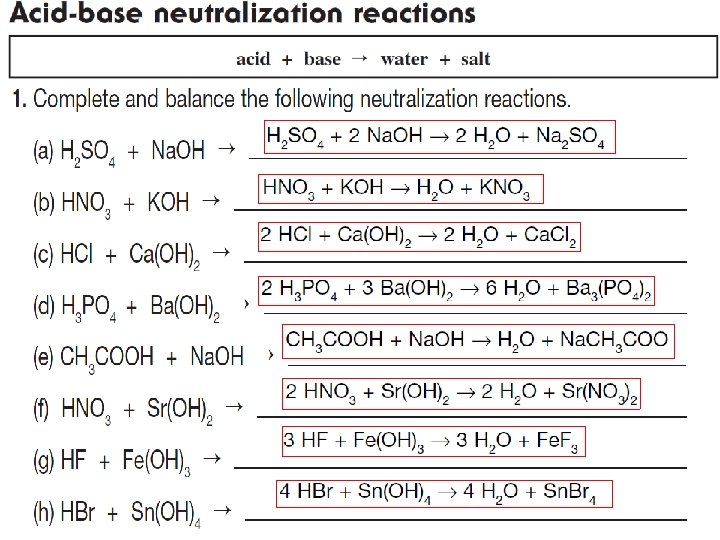
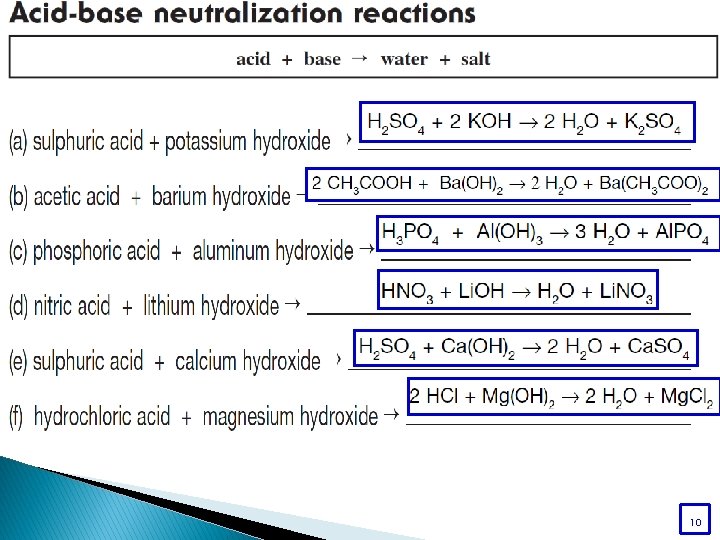
Acid – Base Neutralization Read page 234 – 236 and do:

Acid – Base Neutralization WORKBOO K page 91

acid base acid salt base salt acid salt acid base salt


10

Metal Oxides • Metals react with oxygen to form metal oxides Metal Oxides Na 2 O K 2 O Ca. O Mg. O

Non - Metal Oxides Non - Metals react with oxygen to form non metal oxides Non - Metal Oxides CO 2 SO 2 NO CO

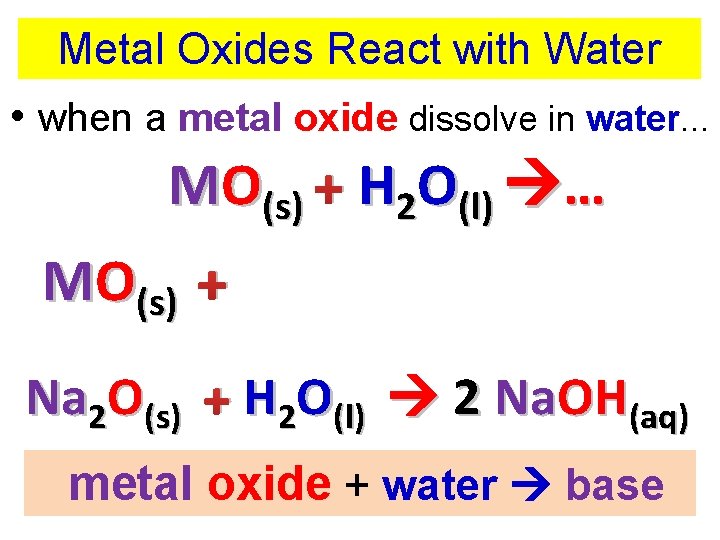
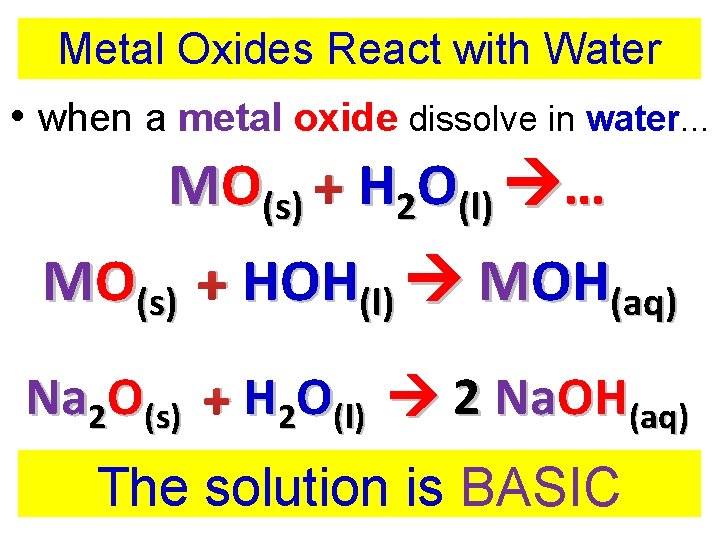
Metal Oxides React with Water • when a metal oxide dissolve in water. . . MO(s) + H 2 O(l) … MO(s) + HOH(l) MOH(aq) Na 2 O(s) + H 2 O(l) 2 Na. OH(aq) metal oxide + water base

Metal Oxides React with Water • when a metal oxide dissolve in water. . . MO(s) + H 2 O(l) … MO(s) + HOH(l) MOH(aq) Na 2 O(s) + H 2 O(l) 2 Na. OH(aq) The solution is BASIC

Non - Metal Oxides React with Water • when a non - metal oxide dissolve in water. . . NO(s) + H 2 O(l) … non - metal oxide + water acid SO 2(s) + H 2 O(l) 2 H 2 SO 3(aq) The solution is ACIDIC

Metal Oxides and Non – Metal Oxides Read page 237 and do:

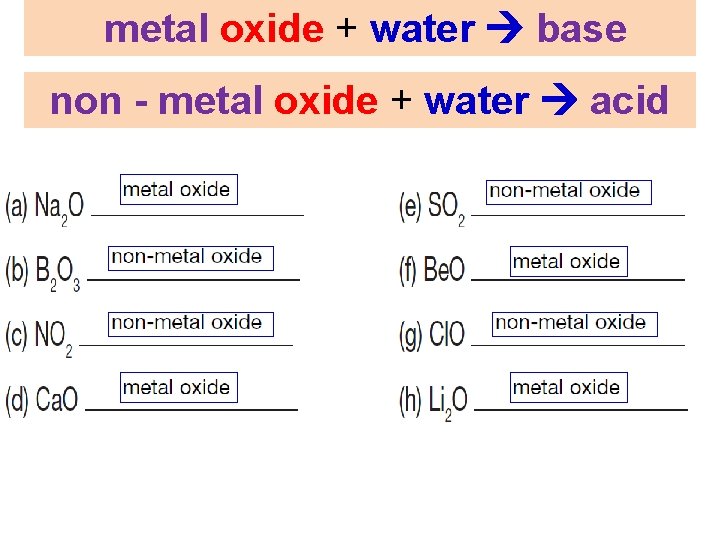
Metal Oxides and Non – Metal Oxides WORKBOO K page 93 metal oxide + water base non - metal oxide + water acid

metal oxide + water base non - metal oxide + water acid 18

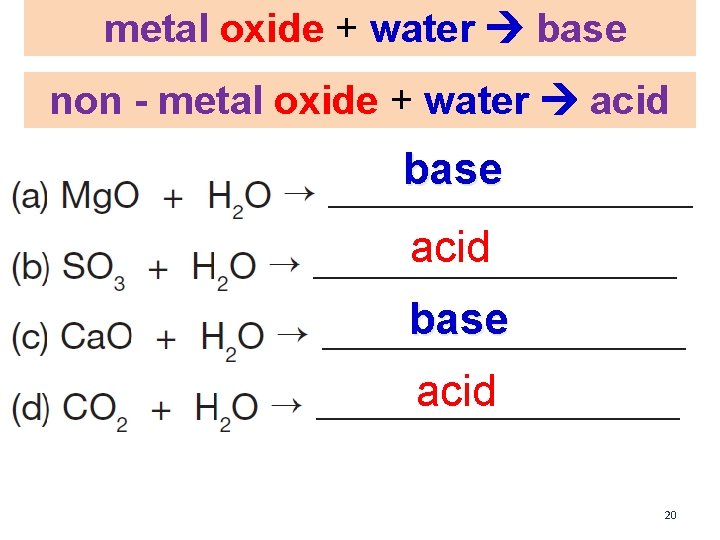
metal oxide + water base non - metal oxide + water acid

metal oxide + water base non - metal oxide + water acid base acid 20

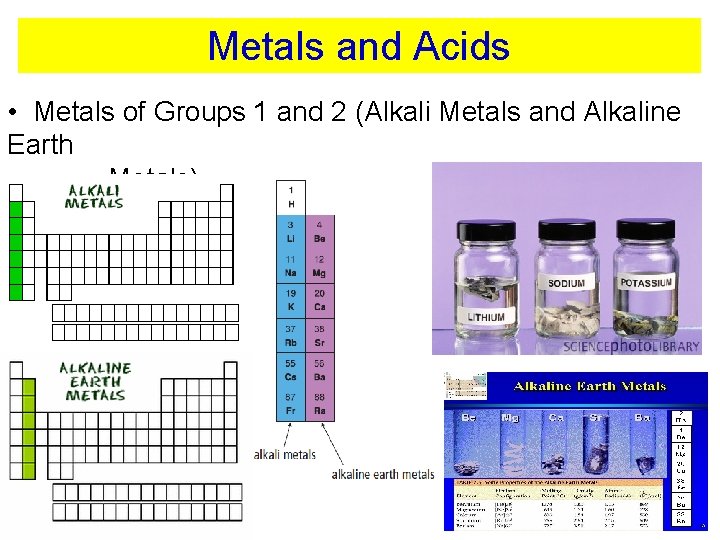
Metals and Acids • Metals of Groups 1 and 2 (Alkali Metals and Alkaline Earth Metals)

Metals and Acids • Metals of Groups 1 and 2 (Alkali Metals and Alkaline Earth Metals) react vigorously with water and acids • Other metals are not that reactive with acids

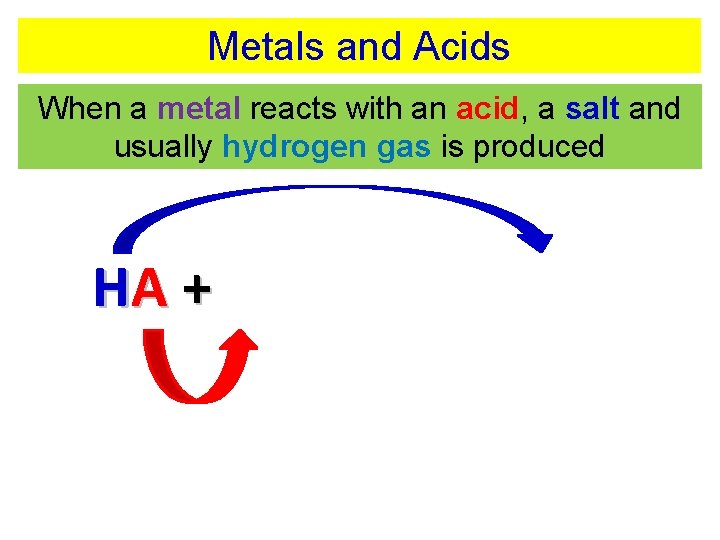
Metals and Acids When a metal reacts with an acid, a salt and usually hydrogen gas is produced HA + M MA + H 2 acid + metal salt + hydrogen

Metals and Acids When a metal reacts with an acid, a salt and usually hydrogen gas is produced HA + M MA + H 2 HCl(aq) + Mg(s) Mg. Cl 2(aq) + H 2(g)

Metals and Acids Read page 238 and do:

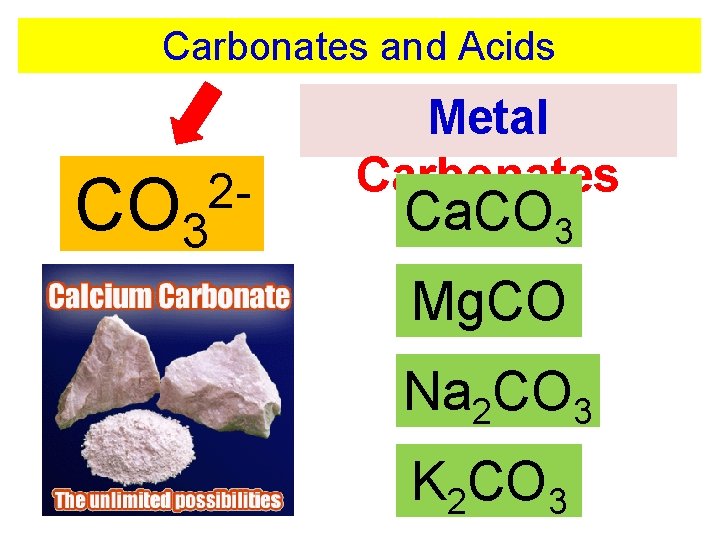
Carbonates and Acids CO 3 2 - Metal Carbonates Ca. CO 3 Mg. CO 3 Na 2 CO 3 K 2 CO 3

Carbonates and Acids Carbonate rocks react with acids to produce. . . CO 3 2 - metal carbonate + acid salt + water + carbon dioxide

Carbonates and Acids Carbonate rocks react with acids to produce. . . CO 3 2 - Ca. CO 3 + H 2 SO 4 Ca. SO 4 + H 2 O + CO 2

5. 2 Check Your Understanding TEXTBOOK page 93

5. 2 SALTS QUIZ • Date: Wednesday, Dec 18
 Insidan region jh
Insidan region jh Kristine nutt
Kristine nutt Chapter 19 acids bases and salts
Chapter 19 acids bases and salts Zn chemistry
Zn chemistry In addition to moving horizontally ocean water moves
In addition to moving horizontally ocean water moves Examples of decomposition reaction
Examples of decomposition reaction Arrhenius acid
Arrhenius acid 2282021
2282021 Urine contains
Urine contains Acid and base characteristics
Acid and base characteristics Chapter 19 acids and bases worksheet answers
Chapter 19 acids and bases worksheet answers Carbonate salts examples
Carbonate salts examples Salt preparation methods
Salt preparation methods Naming salts
Naming salts Functions of bile
Functions of bile Neutralization reaction example
Neutralization reaction example Write the probable colour of the following salts
Write the probable colour of the following salts Acidic salt examples
Acidic salt examples Pure planet earth
Pure planet earth Chapter 19 acids bases and salts
Chapter 19 acids bases and salts Neutral salts
Neutral salts Rectal dosage form example
Rectal dosage form example Hexadentate ligand
Hexadentate ligand White rush bath salts
White rush bath salts Primary aliphatic amines
Primary aliphatic amines Planting media
Planting media Urine formation video
Urine formation video Bile salts in urine
Bile salts in urine Chlor test csn
Chlor test csn Base water
Base water Bile salts names
Bile salts names