Chapter 19 Acids Bases and Salts 1 Section

Chapter 19 Acids, Bases, and Salts 1

Section 19. 1 - Acid-Base Theories • Objectives : – Define the properties of acids and bases. – Compare and contrast acids and bases as defined by theories of Arrhenius and others 2

Properties of Acids • Taste tart or sour (don’t try this at home). – Vinegar – ethanoic acid – Lemon – citric acid • Conduct electricity when in aqueous solution. – Can be strong or weak electrolytes in aqueous solution • React with metals to form H 2 gas. • React with bases (hydroxides) to form water and a salt. 3

Properties of Acids Blue litmus paper turns red in contact with an acid. 4
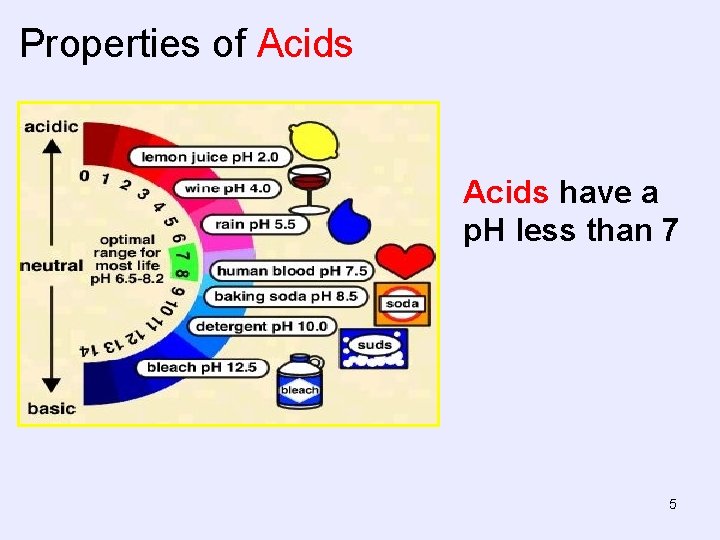
Properties of Acids have a p. H less than 7 5

Properties of Bases • Taste bitter. – Definitely do not try this at home! • Feel slippery • Can be strong or weak electrolytes in aqueous solution • React with acids to form water and a salt • Example of Bases • Sodium hydroxide (lye or Drano), Na. OH • Magnesium hydroxide, Mg(OH)2 - Milk of Magnesia • Calcium hydroxide (lime water), Ca(OH)2 6

Properties of Bases Red litmus paper turns blue in contact with a base. Phenolphthalein turns pink in a base. 7
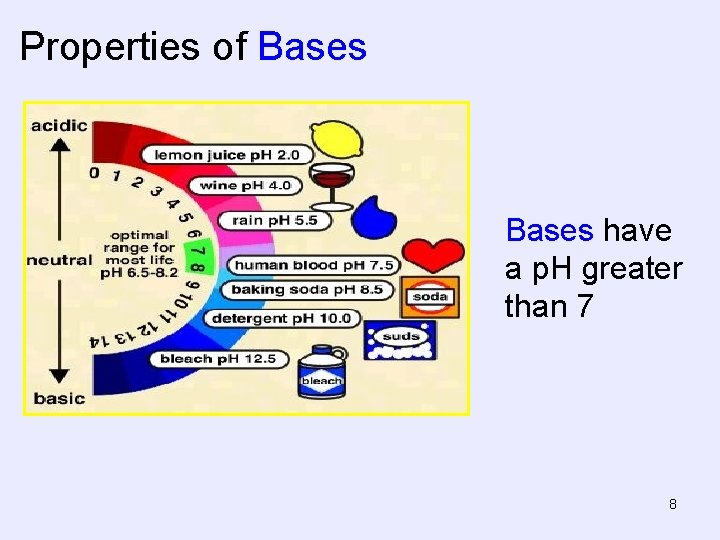
Properties of Bases have a p. H greater than 7 8
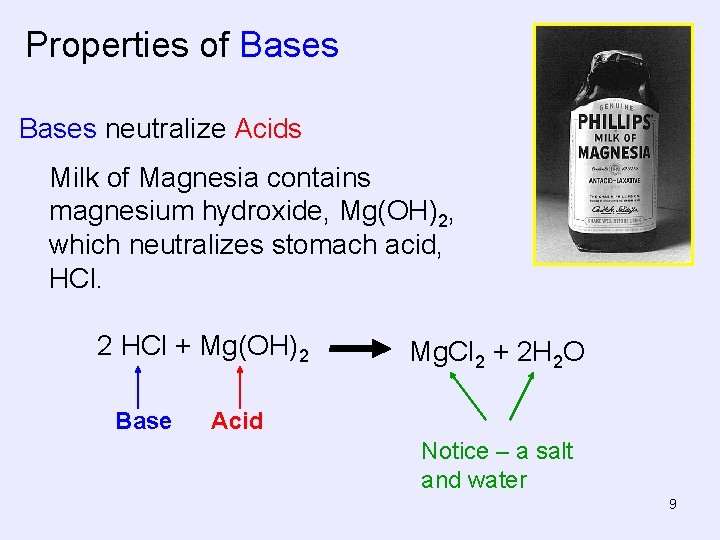
Properties of Bases neutralize Acids Milk of Magnesia contains magnesium hydroxide, Mg(OH)2, which neutralizes stomach acid, HCl. 2 HCl + Mg(OH)2 Base Mg. Cl 2 + 2 H 2 O Acid Notice – a salt and water 9

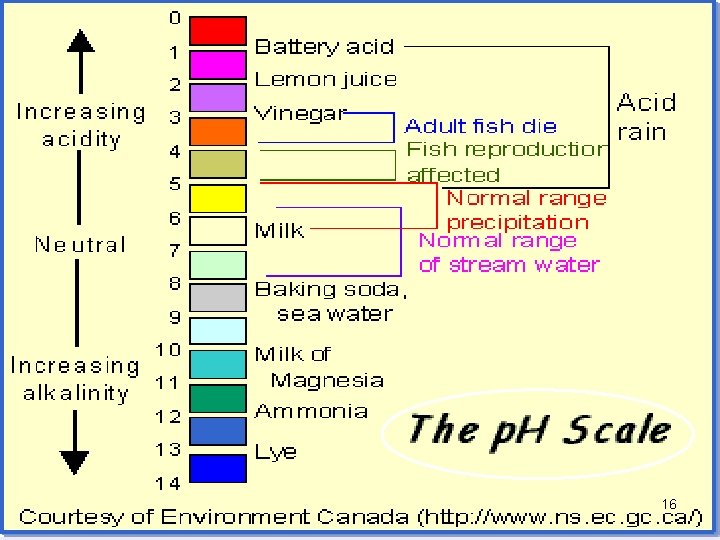
• Acids & Bases Chart 10

19. 2 11

Section 19. 2 - Hydrogen Ions and Acidity • Objectives – Describe how [H 1+] and [OH 1 -] are related in an aqueous solution. – Classify a solution as neutral, acidic, or basic given the hydrogen-ion or hydroxide-ion concentration. – Convert hydrogen-ion concentrations into p. H values and hydroxide-ion concentrations into p. OH values. – Describe the purpose of an acid-base indicator. 12
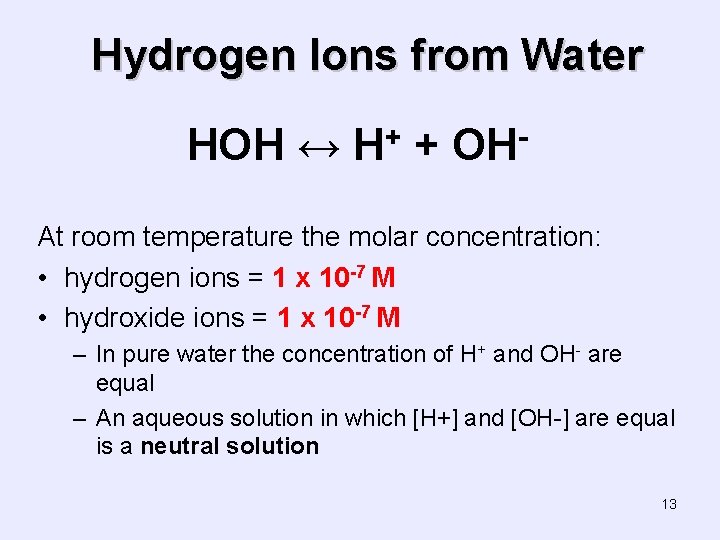
Hydrogen Ions from Water HOH ↔ + H + OH At room temperature the molar concentration: • hydrogen ions = 1 x 10 -7 M • hydroxide ions = 1 x 10 -7 M – In pure water the concentration of H+ and OH- are equal – An aqueous solution in which [H+] and [OH-] are equal is a neutral solution 13
![Hydrogen Ions from Water HOH ↔ H+ + OH– Acidic solution • [H+] is Hydrogen Ions from Water HOH ↔ H+ + OH– Acidic solution • [H+] is](http://slidetodoc.com/presentation_image_h/0c6dac2a8331eb9614d7df7a0acbde50/image-14.jpg)
Hydrogen Ions from Water HOH ↔ H+ + OH– Acidic solution • [H+] is greater than [OH-] • [H+] of an acidic solution is greater than 1 x 10 -7 M – Basic solution (AKA alkaline solution) • [H+] is less than [OH-] • [H+] of an alkaline solution is less than 1 x 10 -7 M 14
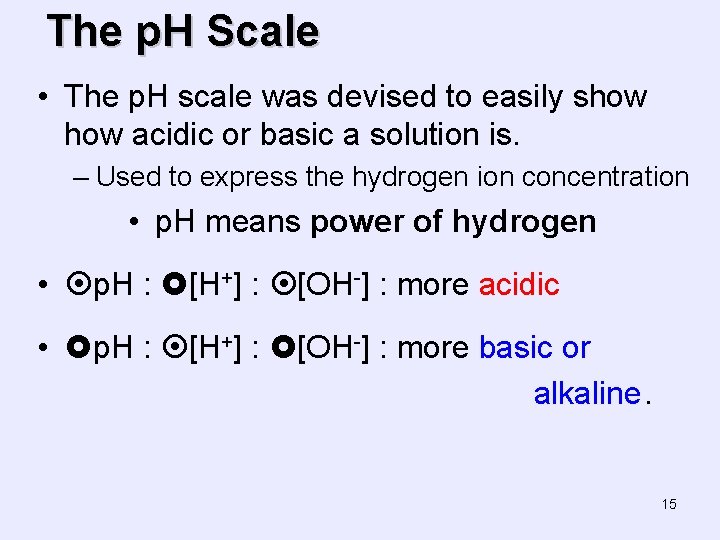
The p. H Scale • The p. H scale was devised to easily show acidic or basic a solution is. – Used to express the hydrogen ion concentration • p. H means power of hydrogen • p. H : [H+] : [OH-] : more acidic • p. H : [H+] : [OH-] : more basic or alkaline. 15
16
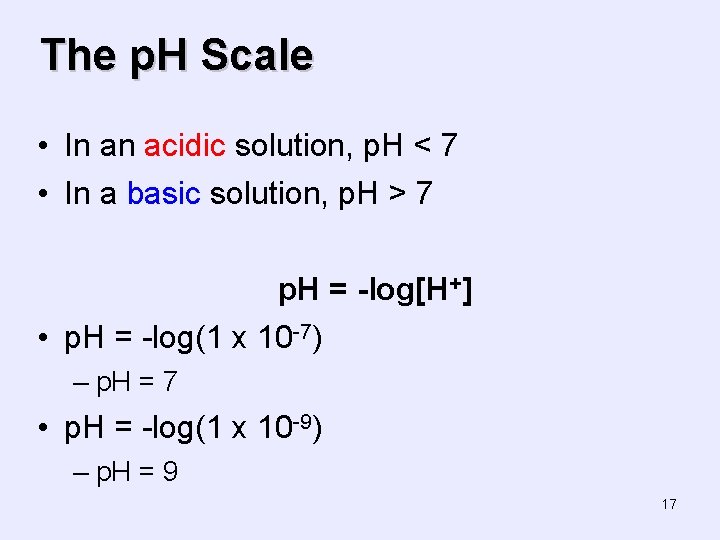
The p. H Scale • In an acidic solution, p. H < 7 • In a basic solution, p. H > 7 p. H = -log[H+] • p. H = -log(1 x 10 -7) – p. H = 7 • p. H = -log(1 x 10 -9) – p. H = 9 17
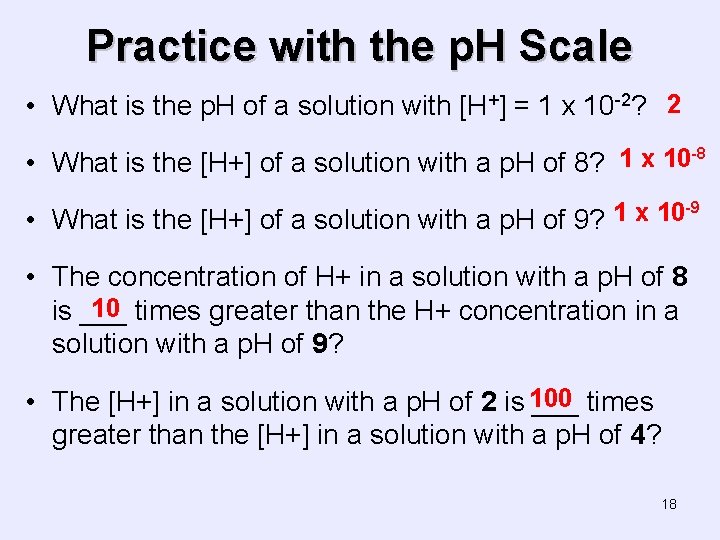
Practice with the p. H Scale • What is the p. H of a solution with [H+] = 1 x 10 -2? 2 • What is the [H+] of a solution with a p. H of 8? 1 x 10 -8 -9 1 x 10 • What is the [H+] of a solution with a p. H of 9? • The concentration of H+ in a solution with a p. H of 8 10 times greater than the H+ concentration in a is ___ solution with a p. H of 9? • The [H+] in a solution with a p. H of 2 is 100 ___ times greater than the [H+] in a solution with a p. H of 4? 18
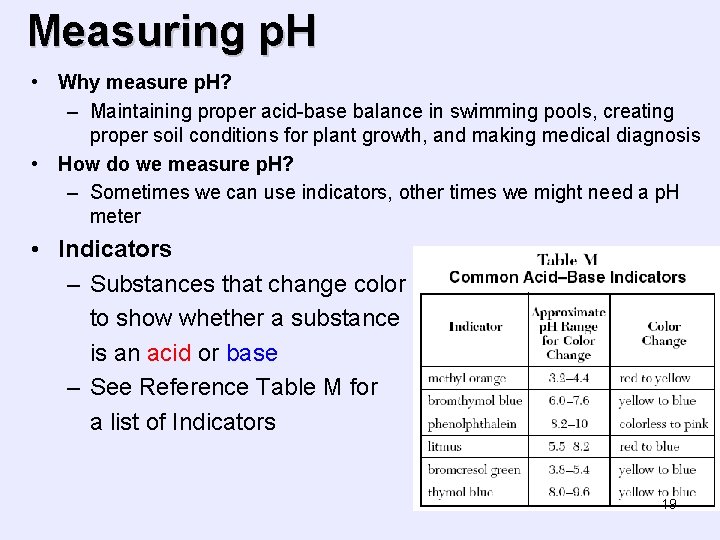
Measuring p. H • Why measure p. H? – Maintaining proper acid-base balance in swimming pools, creating proper soil conditions for plant growth, and making medical diagnosis • How do we measure p. H? – Sometimes we can use indicators, other times we might need a p. H meter • Indicators – Substances that change color to show whether a substance is an acid or base – See Reference Table M for a list of Indicators 19
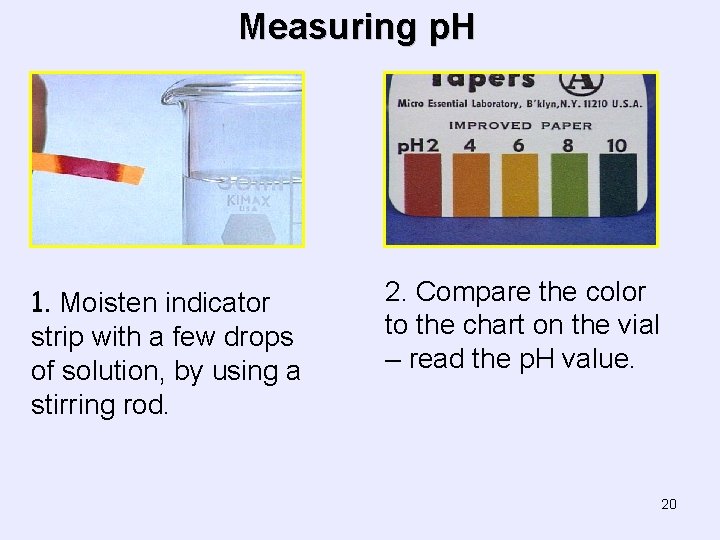
Measuring p. H 1. Moisten indicator strip with a few drops of solution, by using a stirring rod. 2. Compare the color to the chart on the vial – read the p. H value. 20

19. 4 21

Section 19. 4 - Neutralization Reactions • Objectives: – Define the products of an acid-base reaction. – Explain how acid-base titration is used to calculate the concentration of an acid or a base. – Explain the concept of equivalence in neutralization reactions. – Describe the relationship between equivalence point and the end point of a titration. 22
![Neutralization Reactions • Remember, in a neutral solution [H+] = [OH-] • So, if Neutralization Reactions • Remember, in a neutral solution [H+] = [OH-] • So, if](http://slidetodoc.com/presentation_image_h/0c6dac2a8331eb9614d7df7a0acbde50/image-23.jpg)
Neutralization Reactions • Remember, in a neutral solution [H+] = [OH-] • So, if I mix a strong acid containing a certain number of H+ ions with strong base that has an equal number of OH- ions, a neutral solution results Strong Acid with: Strong Base with: 1000 H+ ions 1000 OH- ions [H+] = [OH-]: Neutral solution 23
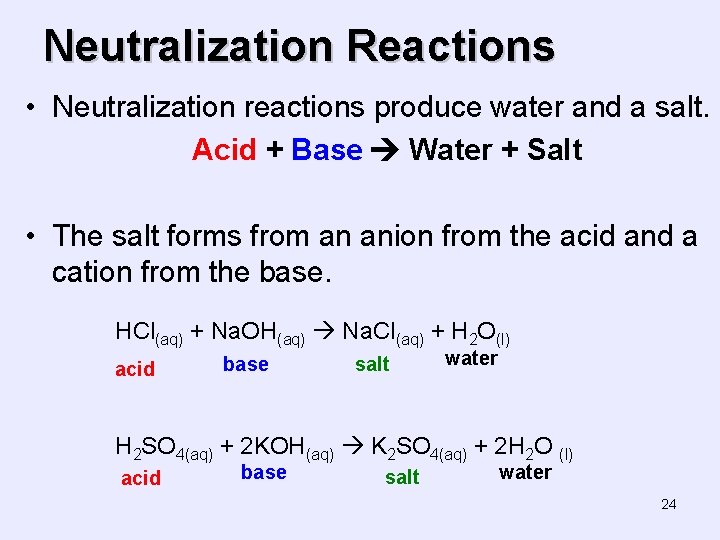
Neutralization Reactions • Neutralization reactions produce water and a salt. Acid + Base Water + Salt • The salt forms from an anion from the acid and a cation from the base. HCl(aq) + Na. OH(aq) Na. Cl(aq) + H 2 O(l) acid base salt water H 2 SO 4(aq) + 2 KOH(aq) K 2 SO 4(aq) + 2 H 2 O (l) acid base salt water 24
Applications of Neutralization Reactions – Properties related to every day: • Antacids depend on neutralization • Farmers use these reactions to control soil p. H • Human body kidney stones from insoluble salts from neutralization reactions Acid + Base Water + Salt 25

Titration • We use neutralization reactions to determine the concentration of an acid (or base) in a solution. • Remember that indicators indicate the p. H by the color they turn. – Often we use phenolphthalein • It is colorless in neutral and acidic solutions • Pink in basic solutions 26
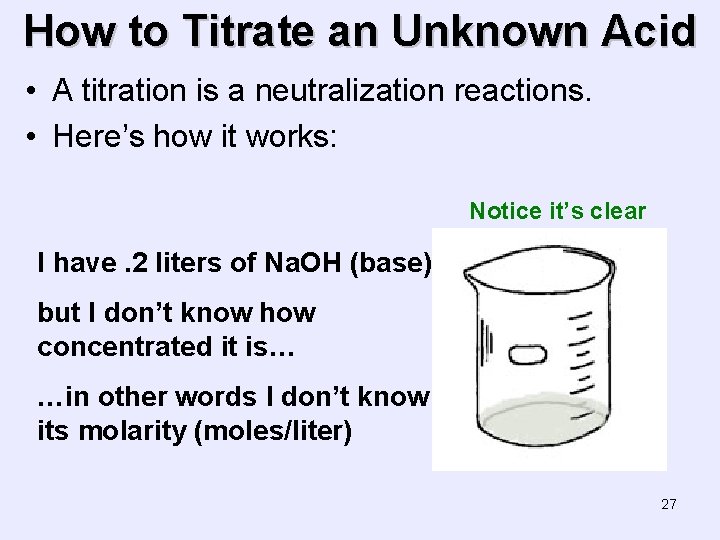
How to Titrate an Unknown Acid • A titration is a neutralization reactions. • Here’s how it works: Notice it’s clear I have. 2 liters of Na. OH (base) but I don’t know how concentrated it is… …in other words I don’t know its molarity (moles/liter) 27
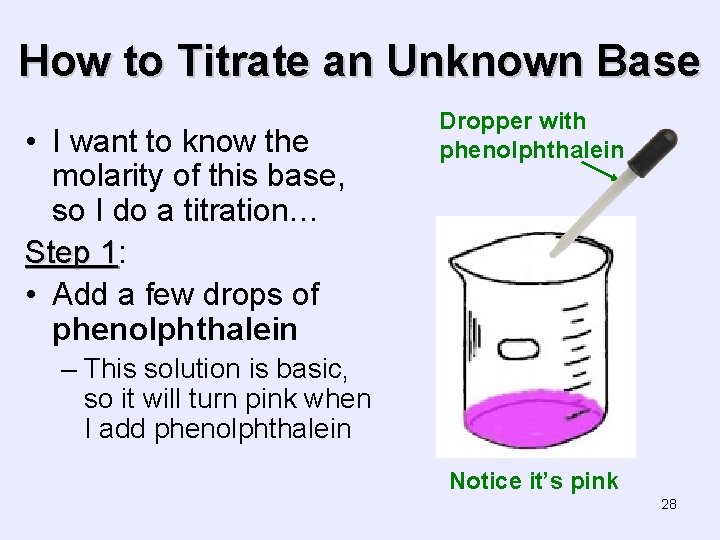
How to Titrate an Unknown Base • I want to know the molarity of this base, so I do a titration… Step 1: 1 • Add a few drops of phenolphthalein Dropper with phenolphthalein – This solution is basic, so it will turn pink when I add phenolphthalein Notice it’s pink 28
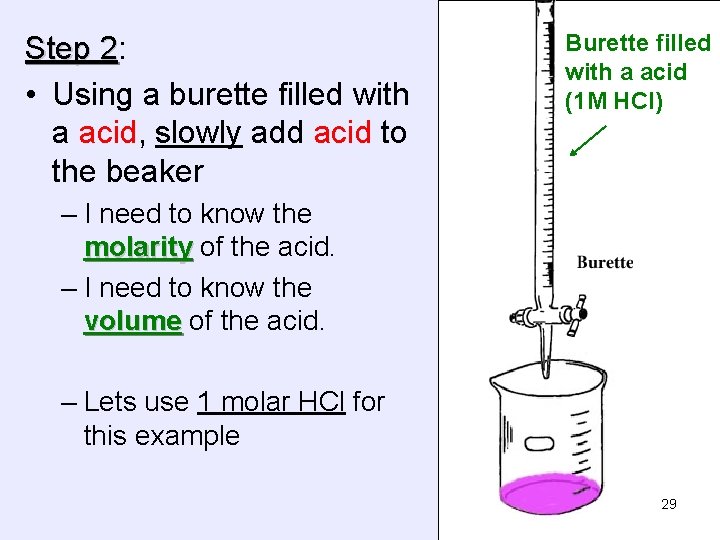
Step 2: 2 • Using a burette filled with a acid, slowly add acid to the beaker Burette filled with a acid (1 M HCl) – I need to know the molarity of the acid. – I need to know the volume of the acid. – Lets use 1 molar HCl for this example 29
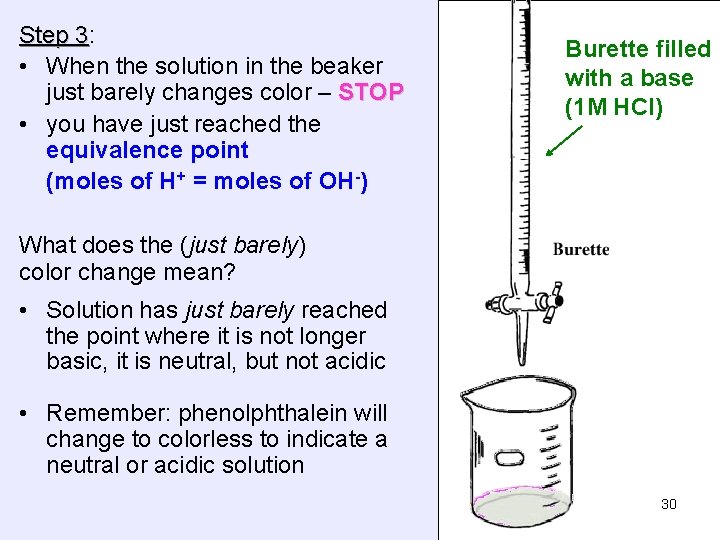
Step 3: 3 • When the solution in the beaker just barely changes color – STOP • you have just reached the equivalence point (moles of H+ = moles of OH-) Burette filled with a base (1 M HCl) What does the (just barely) color change mean? • Solution has just barely reached the point where it is not longer basic, it is neutral, but not acidic • Remember: phenolphthalein will change to colorless to indicate a neutral or acidic solution 30
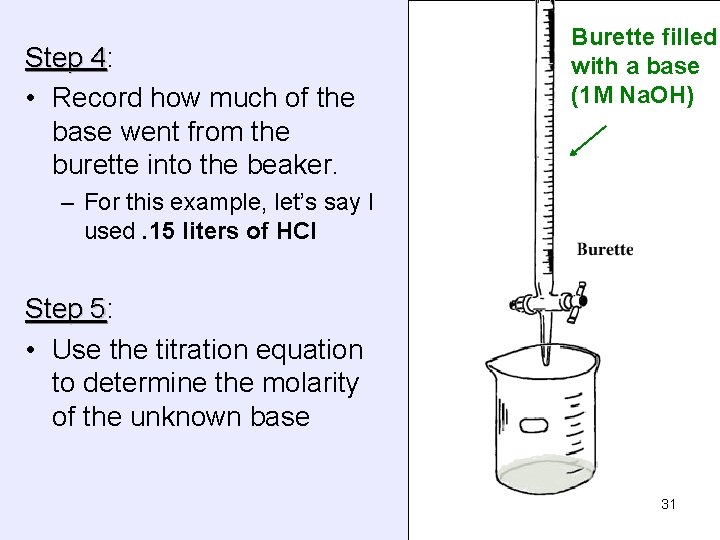
Step 4: 4 • Record how much of the base went from the burette into the beaker. Burette filled with a base (1 M Na. OH) – For this example, let’s say I used. 15 liters of HCl Step 5: 5 • Use the titration equation to determine the molarity of the unknown base 31
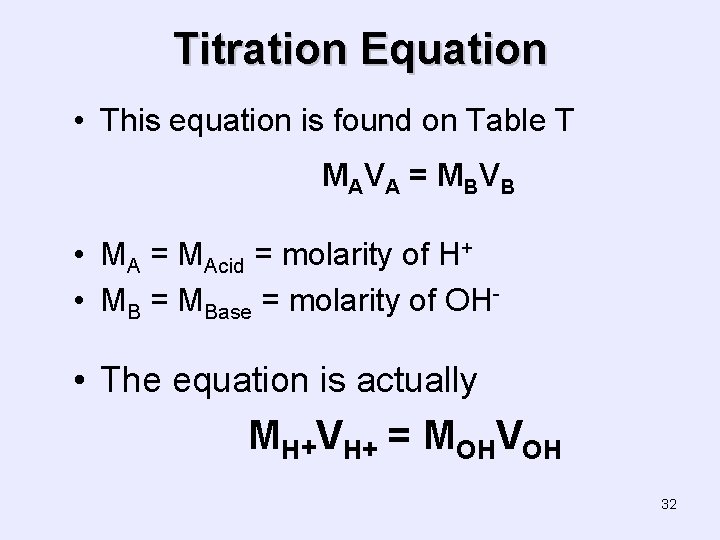
Titration Equation • This equation is found on Table T MA V A = M B V B • MA = MAcid = molarity of H+ • MB = MBase = molarity of OH- • The equation is actually MH+VH+ = MOHVOH 32
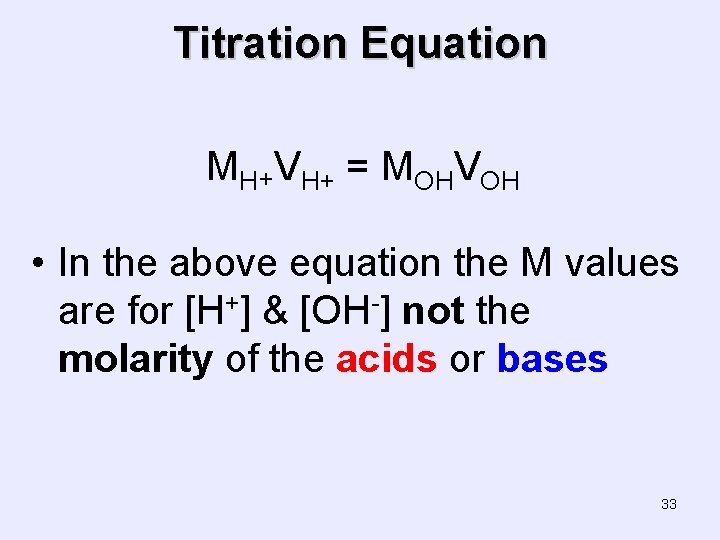
Titration Equation MH+VH+ = MOHVOH • In the above equation the M values are for [H+] & [OH-] not the molarity of the acids or bases 33
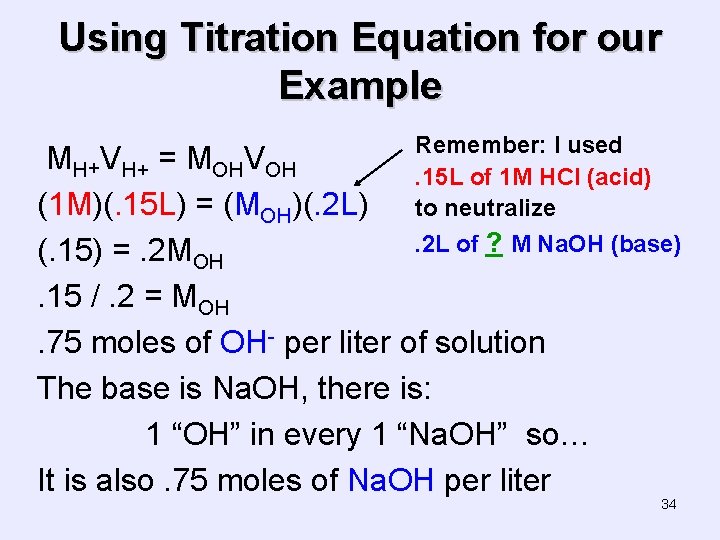
Using Titration Equation for our Example Remember: I used. 15 L of 1 M HCl (acid) to neutralize MH+VH+ = MOHVOH (1 M)(. 15 L) = (MOH)(. 2 L). 2 L of ? M Na. OH (base) (. 15) =. 2 MOH. 15 /. 2 = MOH. 75 moles of OH- per liter of solution The base is Na. OH, there is: 1 “OH” in every 1 “Na. OH” so… It is also. 75 moles of Na. OH per liter 34
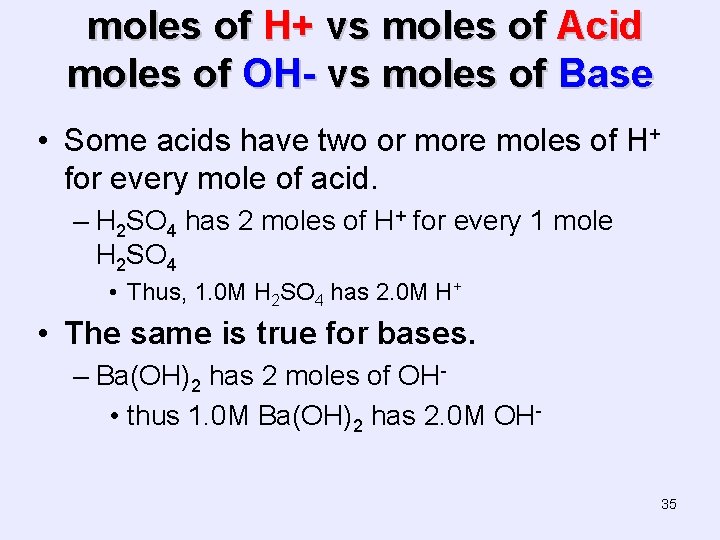
moles of H+ vs moles of Acid moles of OH- vs moles of Base • Some acids have two or more moles of H+ for every mole of acid. – H 2 SO 4 has 2 moles of H+ for every 1 mole H 2 SO 4 • Thus, 1. 0 M H 2 SO 4 has 2. 0 M H+ • The same is true for bases. – Ba(OH)2 has 2 moles of OH • thus 1. 0 M Ba(OH)2 has 2. 0 M OH 35
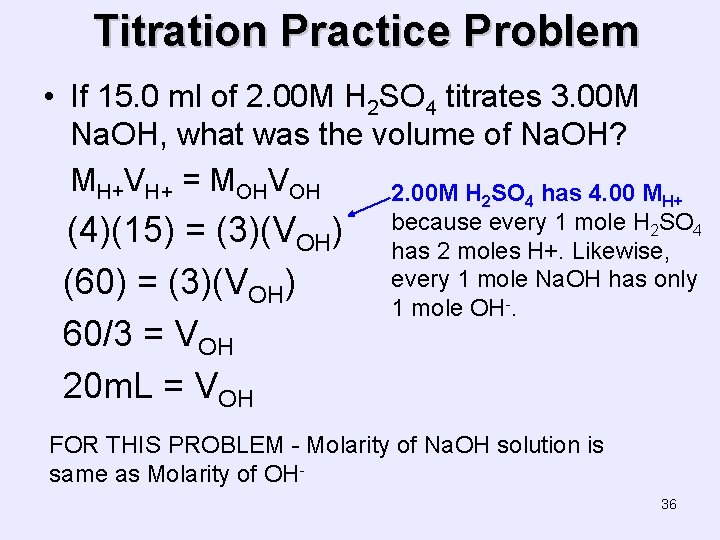
Titration Practice Problem • If 15. 0 ml of 2. 00 M H 2 SO 4 titrates 3. 00 M Na. OH, what was the volume of Na. OH? MH+VH+ = MOHVOH 2. 00 M H 2 SO 4 has 4. 00 MH+ (4)(15) = (3)(VOH) (60) = (3)(VOH) 60/3 = VOH 20 m. L = VOH because every 1 mole H 2 SO 4 has 2 moles H+. Likewise, every 1 mole Na. OH has only 1 mole OH-. FOR THIS PROBLEM - Molarity of Na. OH solution is same as Molarity of OH 36

37

Titration Practice Problem • If 25. 0 ml of 2. 00 M HCl titrates 30. 0 ml of Na. OH, what is the molar concentration of Na. OH? 2. 00 M HCl has 2. 00 MH+ because every 1 mole HCl has 1 mole H+. Likewise, every 1 mole Na. OH has 1 mole OH-. FOR THIS PROBLEM - Molarity of solution is same as Molarity of H+ and OHANSWER: 1. 67 MOH- = 1. 67 moles OH-/ 1 Liter 38

Videos • • Intro video (1: 24 min): http: //www. youtube. com/watch? v=S 7 Cs. O 3 p 5 t 5 M Acids & Bases with Hannah & Kelsie (~3 min): http: //www. youtube. com/watch? v=6 f. Z 2 OJ-HNr 0&feature=related • • Puppet Acid & base Video(6: 56 min): http: //www. youtube. com/watch? v=4 c. L 1 kmj. ASb. Y Richard Thorpe Acids & Bases: (~3: 30 min): http: //www. youtube. com/watch? v=JCcp. WMW-Sz. Y&feature=related Water as an Acid & Base(8: 44): http: //www. youtube. com/watch? v=KSs. S 1 jk. BMn. U • Rainbow Connection with the Muppets(~3: 22 min): (man) http: //www. youtube. com/watch? v=l. Cr. MB 8341 r. U • (female) http: //www. youtube. com/watch? v=IUAa 0 us. LZe. U&feature=related • Acid / base Indicators: http: //www. youtube. com/watch? v=6 Y 4 Y-__ME 60 (4: 11 min) • • • How to do a Titration Experiment(~2 min): http: //www. youtube. com/watch? v=r 1 u. EXOOR 2 n. U&feature=related World of Chemistry (the Proton in Chemistry (29: 05 min): http: //www. learner. org/resources/series 61. html# Neutralization reaction: http: //www. youtube. com/watch? v=8 IRI 5 g. PR 5 EY (59 sec 0 Acid / base Neutralization: http: //www. youtube. com/watch? v=en. PG 0 vkn 4 QA (3: 23 min) Acid & Bases have two different Faces: http: //www. youtube. com/watch? v=z. TLi. JE-j 1 -I (3: 26 min) Kid mixing Vinegar & Baking Soda - http: //www. youtube. com/watch? v=3 hor 1 se. Pkf. I (55 sec) Titrating an Acid http: //www. youtube. com/watch? v=6 wo. C_HSVtm. Q (3: 22 min) Titration of an Acid http: //www. youtube. com/watch? v=y. NSE_xa 5 lf. A (1: 22 min) • • How to Perform a titration: http: //www. youtube. com/watch? v=YDzz. Mcrdy. B 4 (6: 07 min) Cool Universal Indicator Video http: //www. youtube. com/watch? v=TXA 9 NNLr. QFc (1: 52 min) 39
- Slides: 39