Determine the Ionic Charge of the following 1











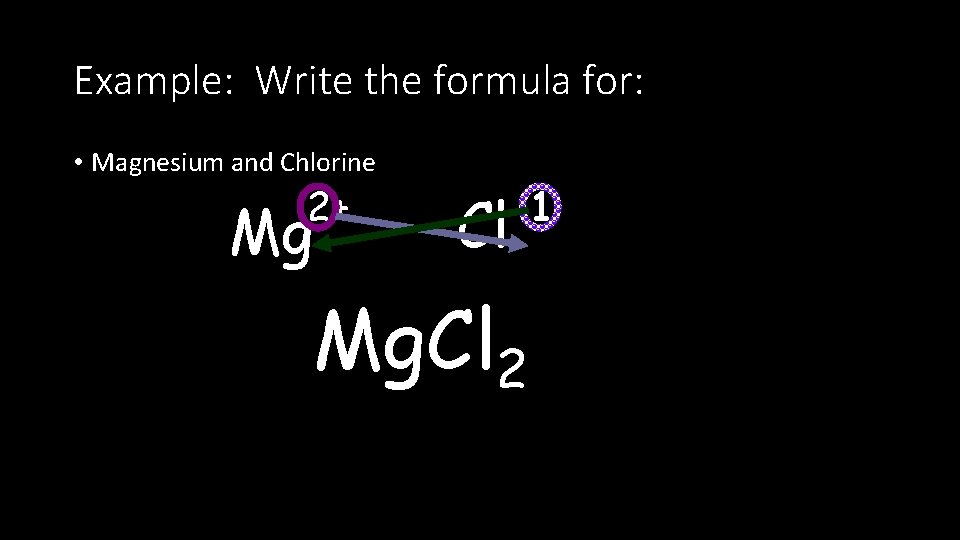














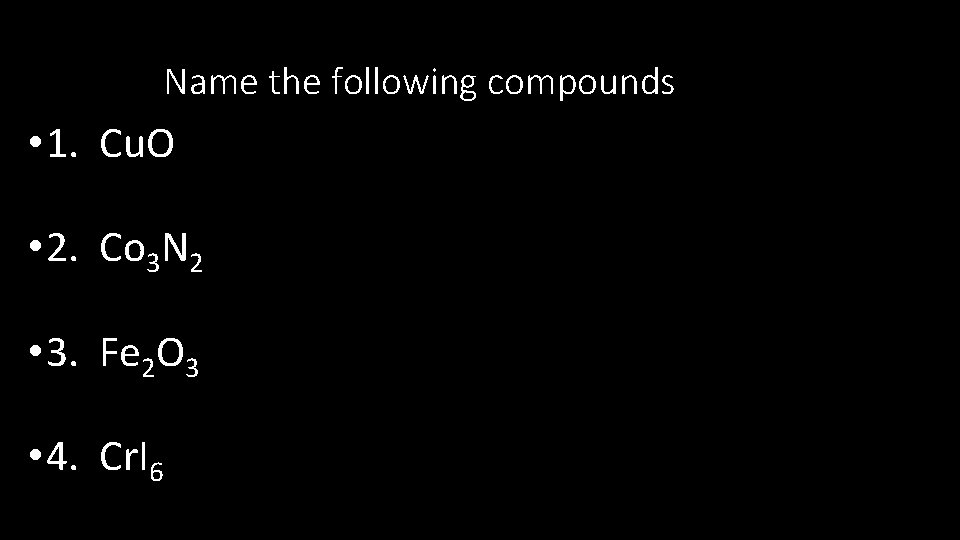


- Slides: 30


Determine the Ionic Charge of the following 1. Sr 2. Te 3. N 4. Mg 5. Se

Compounds • Compounds are a combination of at least two elements that act as one unit • Compounds have separate identities different from their component elements

Compounds • When a compound forms it causes a chemical bond to occur. • A chemical bond -is the force that holds two atoms together • This is the result when electrons are gained, lost, or shared between atoms

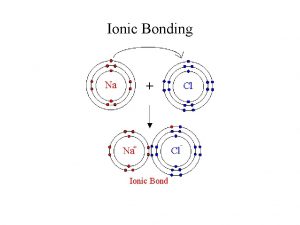
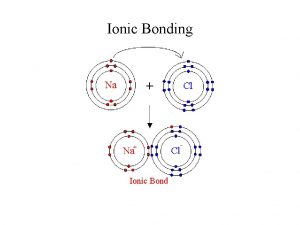
Ionic Bonds • Ionic bonds form between METALS & NONMETALS only • Example: Na. Cl, Mg. Cl 2 • Ionic bonds form ionic compounds which stay together due to the electrostatic attraction between the cat-ion and an-ion • Ionic compounds are usually crystalline solids at room temperature

Chemical Formulas • Chemical Formulas show the kinds and number of each atom present • The small numbers written below a symbol are called SUBSCRIPTS • Subscripts show the number of each atom present • Metals are written first in the formula

Determine the kinds and number of each atom in the following: • 1. • 2. • 3. • 4. • 5. • 6. CO 2 Al(NO 3)3 CCl 4 Mg. SO 4 Mn(NO 3)7 HCl

Charges for Groups 3 -12 • Groups 3 -12 are known collectively as the transition metals • These metals can take various charges • For example: • Cr can take a +2, +3, or +6 charge • Fe can take a +2 or +3 charge

Charges for Groups 3 -12 • Exceptions: • (Three that you need to memorize) • Silver is always +1 • Zinc is always +2 • Cadmium is always +2

Binary Ionic Compounds • A binary ionic compound is an ionic compound that contains only 2 elements • Examples: • Na. Cl Mg. F 2 • Binary ionic compounds use the ionic charges in order to write correct formulas

Writing Correct Chemical Formulas for Binary Ionics: • 1. Determine the charge of each ion • 2. Crisscross the value associated with the charge to the bottom right of the other atom • 3. Reduce the subscripts to the lowest possible terms
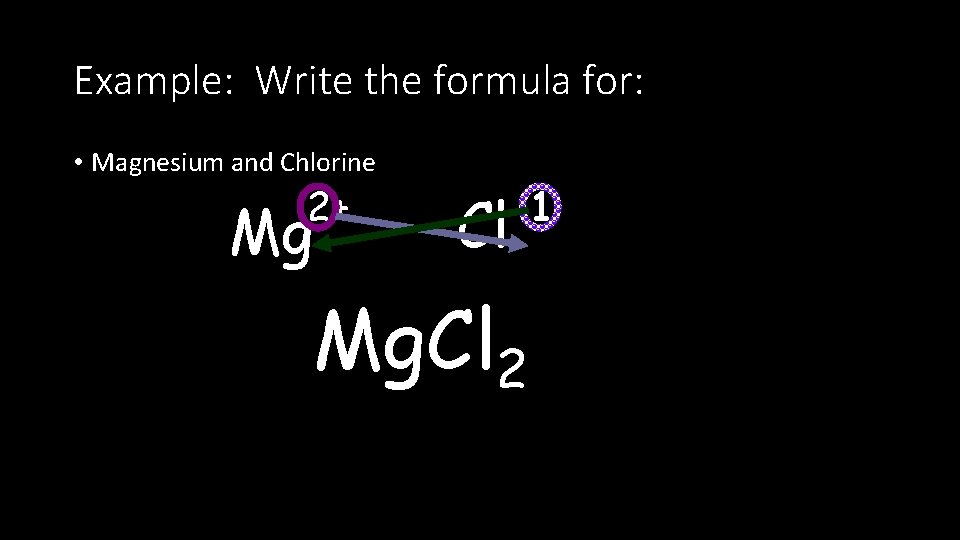
Example: Write the formula for: • Magnesium and Chlorine 2+ Mg -1 Cl Mg. Cl 2

Write Formulas for the following: • 1. Mg, P • 2. Al, S • 3. Ag, Cl • 4. K, I • 5. Zn, P • 6. Na, O • 7. Al, F

Practice Writing Compounds Write the symbols for the following names Ca, Cl • 2. Cd, O • 3. Na, F • 4. Ag, N • 5. Zn, S • 6. Ra, Br • 1.

Practice Writing Compounds • Mg, N • Al, S • Na, N • Cd, F • Ag, P • Sr, Br • Ga, At

• When Sodium and Sulfur react, what compound is produced? • When Calcium and Bromine react, what compound is produced?

Naming Ions • Cations take the metal’s name and add cation • Example: Na+ = sodium cation • Name the following: • K+ • Al 3+

Naming Ions • Anions always add “-ide” as a suffix in place of the original ending • Cl- = Chloride anion (Not Chlorine) • O 2 - = Oxide anion (Not Oxygen) • Name the following: • S 2 • N 3 -

Naming Binary Ionic Compounds • In naming binary ionic compounds, put the cation’s name with the anion’s name • Example: Mg. F 2 • Mg+2 = Magnesium cation • F- = Fluoride anion • Magnesium Fluoride • Example: Na 2 S • Na+= Sodium cation • S-2= Sulfide anion • Sodium Sulfide

Binary Ionic Compounds Containing Transition Metals • In naming compounds with transition metals, include the roman numeral with the transition metal • To figure out the transition metal’s charge, look at the anion’s charge

Binary Ionic Compounds Containing Transition Metals • Example: Fe. Cl 3 • Cl has a -1 charge • There are 3 Cl in the formula • There is only one Fe in the formula • So Fe must have a +3 charge to balance out the Cl charge • Iron (III) Chloride

Naming Transition Metals • The transition metals can take various charges in ionic compounds • In order to name them, the charges must be given • The transition metals are located in groups 3 -12 (the center of the periodic table)

Naming Transition Metals Using The Roman Numeral System • Cu 2+ is called Copper (II) + • Cu is called Copper (I) • Name the following ions: • Fe 2+ , Fe 3+ , Sn 2+ , Sn 4+

Practice Name the following compounds: • 1. Mg. Cl 2 • 2. Li 2 O • 3. Ba. S • 4. Sr 3 P 2 • 5. Rb. Cl • 6. Mg. F 2 • 7. KCl • 8. Cs 2 S • 9. KBr

• Name or write the following compounds • Chromium (VI) Oxide • Ca 3 P 2 • Strontium Sulfide • Cu 2 O

Name or write the correct formula for the following compounds • 1. Zinc Chloride • 2. Fe 3 P 2 • 3. Sodium Selenide • 4. Cr. N 3 • 5. Gold (II) Iodide
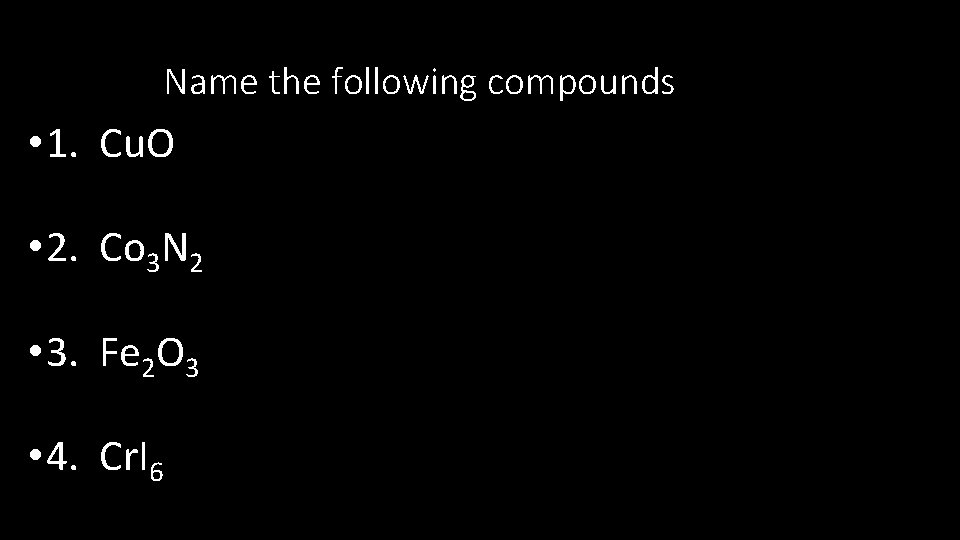
Name the following compounds • 1. Cu. O • 2. Co 3 N 2 • 3. Fe 2 O 3 • 4. Cr. I 6

Write the formula for the following names • Write formulas for: • 1. barium oxide • 2. cobalt (II) fluoride • 3. silver phosphide • 4. zinc chloride • 5. Titanium (IV) oxide

Naming Ternary Compounds • Ternary compounds are named similar to binary ionic compounds: • Still going to be using the cation’s name but this time you are going to be using the polyatomic name as the anion’s name

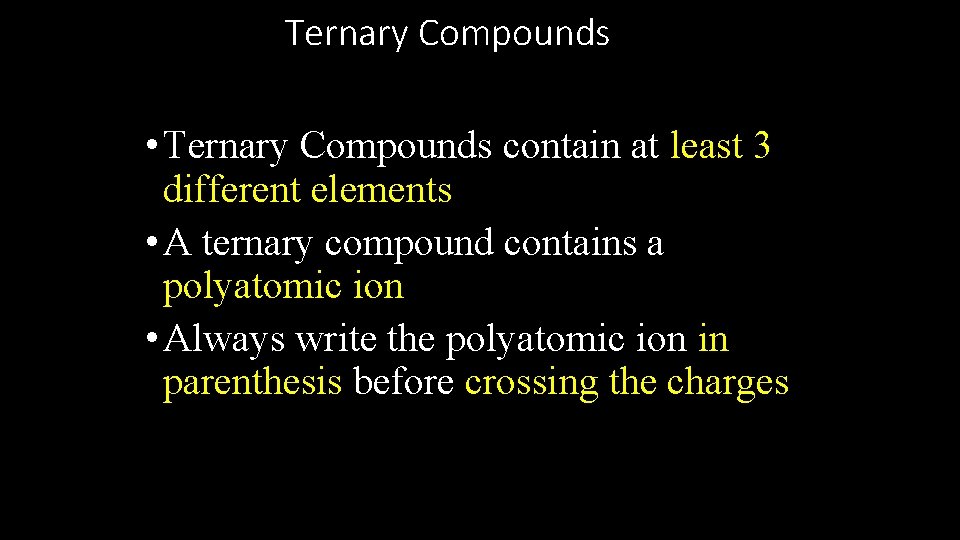
Ternary Compounds • Ternary Compounds contain at least 3 different elements • A ternary compound contains a polyatomic ion • Always write the polyatomic ion in parenthesis before crossing the charges
 Full ionic equation
Full ionic equation Example of cation
Example of cation Ionic binary compounds multiple charge cations
Ionic binary compounds multiple charge cations Trends for ionic charge
Trends for ionic charge Sulfate charge
Sulfate charge Cl-1 ion name
Cl-1 ion name Cations with multiple charges
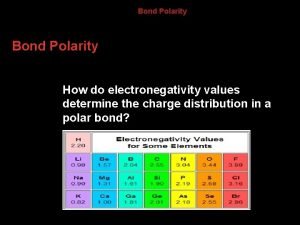
Cations with multiple charges How do electronegativity values determine the charge
How do electronegativity values determine the charge Formal charge resonance
Formal charge resonance Calculating formal charges
Calculating formal charges How to calculate formal charge?
How to calculate formal charge? Difference between charge and electric charge
Difference between charge and electric charge Difference between charge and electric charge
Difference between charge and electric charge Determine whether the following relation is a function.
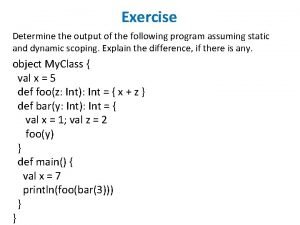
Determine whether the following relation is a function. What is the output of the following program:
What is the output of the following program: Chúa sống lại
Chúa sống lại Hổ đẻ mỗi lứa mấy con
Hổ đẻ mỗi lứa mấy con Diễn thế sinh thái là
Diễn thế sinh thái là đại từ thay thế
đại từ thay thế Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Cong thức tính động năng
Cong thức tính động năng Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Thế nào là mạng điện lắp đặt kiểu nổi
Thế nào là mạng điện lắp đặt kiểu nổi Lời thề hippocrates
Lời thề hippocrates Vẽ hình chiếu đứng bằng cạnh của vật thể
Vẽ hình chiếu đứng bằng cạnh của vật thể Phản ứng thế ankan
Phản ứng thế ankan Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Môn thể thao bắt đầu bằng từ chạy
Môn thể thao bắt đầu bằng từ chạy Hình ảnh bộ gõ cơ thể búng tay
Hình ảnh bộ gõ cơ thể búng tay Sự nuôi và dạy con của hươu
Sự nuôi và dạy con của hươu điện thế nghỉ
điện thế nghỉ