6 4 Lewis Structures and Formal Charge Formal





- Slides: 18

6. 4 Lewis Structures and Formal Charge Formal charge can be used to determine the most plausible Lewis Structure when more than one possibility exists for a compound. Formal charge = valence electrons – associated electrons To determine associated electrons: 1) All the atom’s nonbonding electrons are associated with the atom. 2) Half the atom’s bonding electrons are associated with the atom.

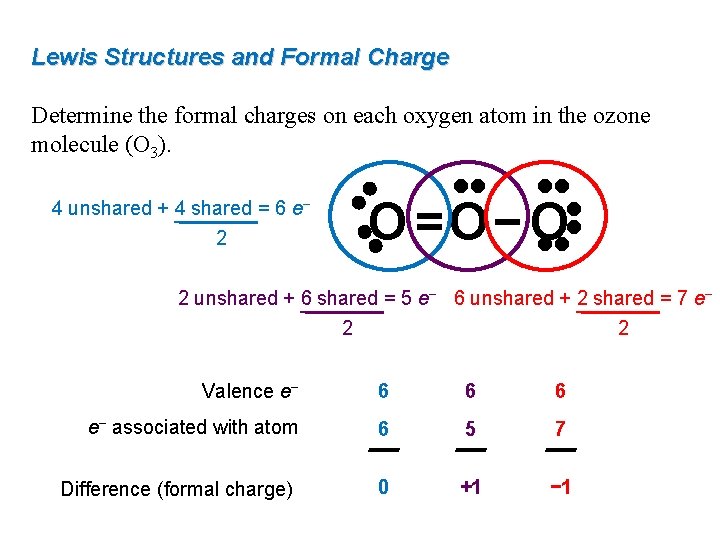
Lewis Structures and Formal Charge Determine the formal charges on each oxygen atom in the ozone molecule (O 3). • • • • O =O− O • • 4 unshared + 4 shared = 6 e− 2 2 unshared + 6 shared = 5 e− 6 unshared + 2 shared = 7 e− 2 2 Valence e− 6 6 6 e− associated with atom 6 5 7 Difference (formal charge) 0 +1 − 1

Worked Example 6. 5 The widespread use of fertilizers has resulted in the contamination of some groundwater with nitrates, which are potentially harmful. Nitrate toxicity is due primarily to its conversion in the body to nitrite (NO 2 -), which interferes with the ability of hemoglobin to transport oxygen. Determine the formal charges on each atom in the nitrate ion (NO 3 -).

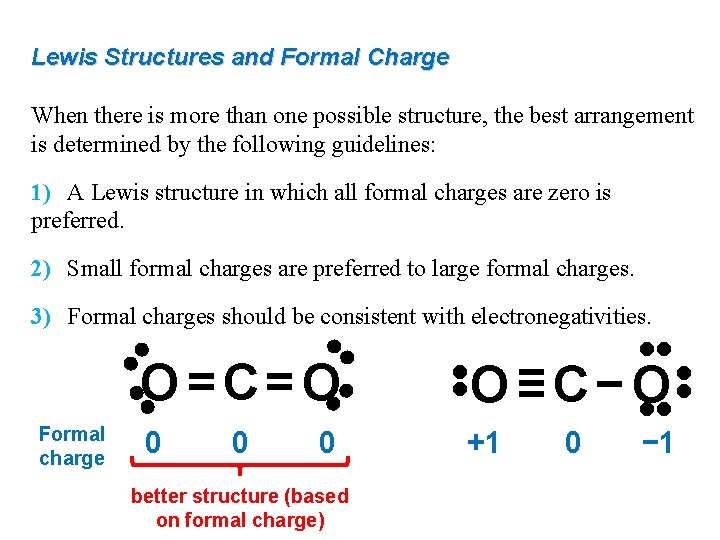
Lewis Structures and Formal Charge When there is more than one possible structure, the best arrangement is determined by the following guidelines: 1) A Lewis structure in which all formal charges are zero is preferred. 2) Small formal charges are preferred to large formal charges. 3) Formal charges should be consistent with electronegativities. • • 0 0 better structure (based on formal charge) +1 • • 0 O≡C−O • • Formal charge • • O=C=O 0 − 1

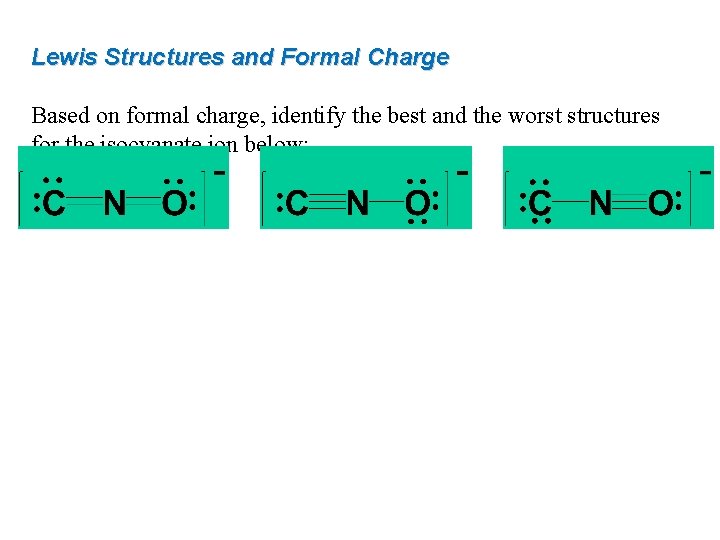
Lewis Structures and Formal Charge Based on formal charge, identify the best and the worst structures for the isocyanate ion below:

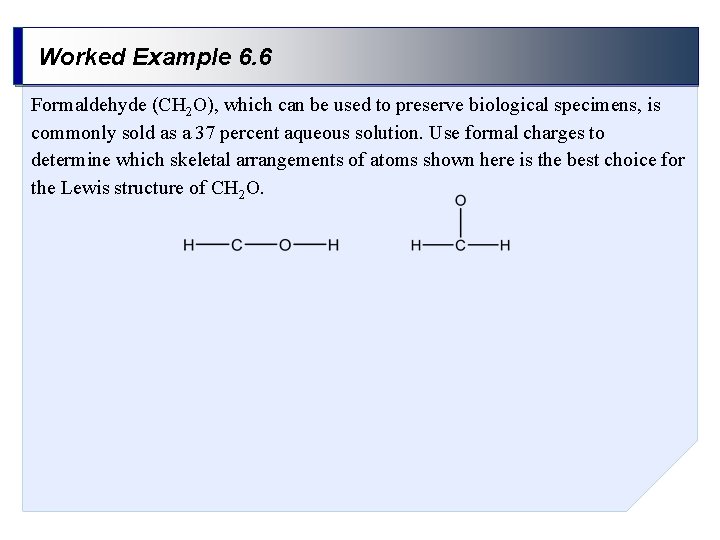
Worked Example 6. 6 Formaldehyde (CH 2 O), which can be used to preserve biological specimens, is commonly sold as a 37 percent aqueous solution. Use formal charges to determine which skeletal arrangements of atoms shown here is the best choice for the Lewis structure of CH 2 O.

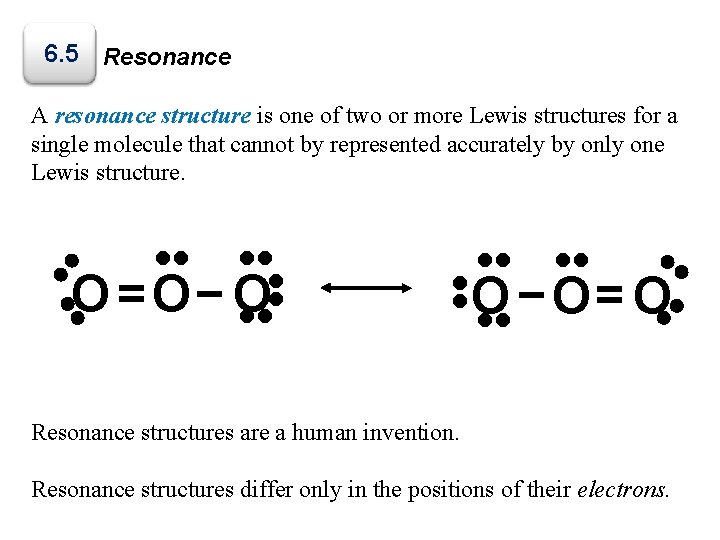
6. 5 Resonance A resonance structure is one of two or more Lewis structures for a single molecule that cannot by represented accurately by only one Lewis structure. • • • • O −O= O • • • • • O =O− O Resonance structures are a human invention. Resonance structures differ only in the positions of their electrons.

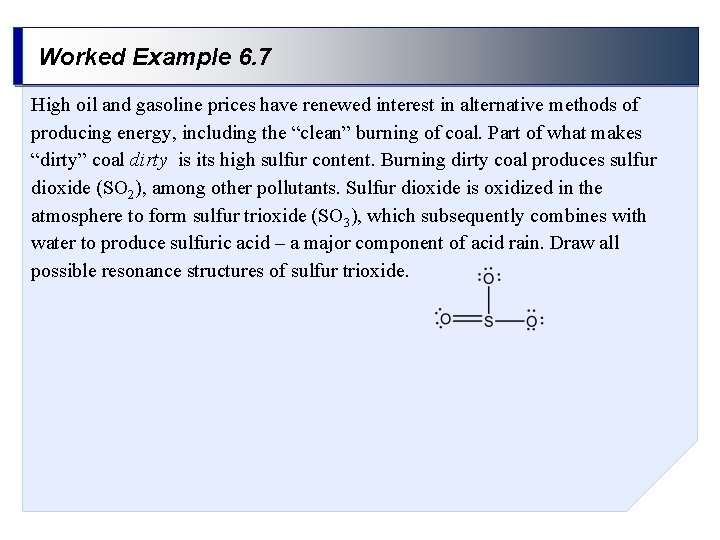
Worked Example 6. 7 High oil and gasoline prices have renewed interest in alternative methods of producing energy, including the “clean” burning of coal. Part of what makes “dirty” coal dirty is its high sulfur content. Burning dirty coal produces sulfur dioxide (SO 2), among other pollutants. Sulfur dioxide is oxidized in the atmosphere to form sulfur trioxide (SO 3), which subsequently combines with water to produce sulfuric acid – a major component of acid rain. Draw all possible resonance structures of sulfur trioxide.

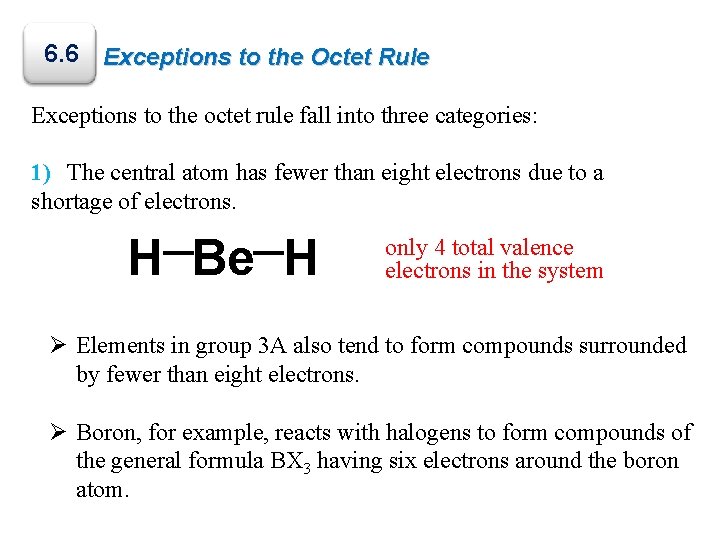
6. 6 Exceptions to the Octet Rule Exceptions to the octet rule fall into three categories: 1) The central atom has fewer than eight electrons due to a shortage of electrons. H Be H only 4 total valence electrons in the system Ø Elements in group 3 A also tend to form compounds surrounded by fewer than eight electrons. Ø Boron, for example, reacts with halogens to form compounds of the general formula BX 3 having six electrons around the boron atom.

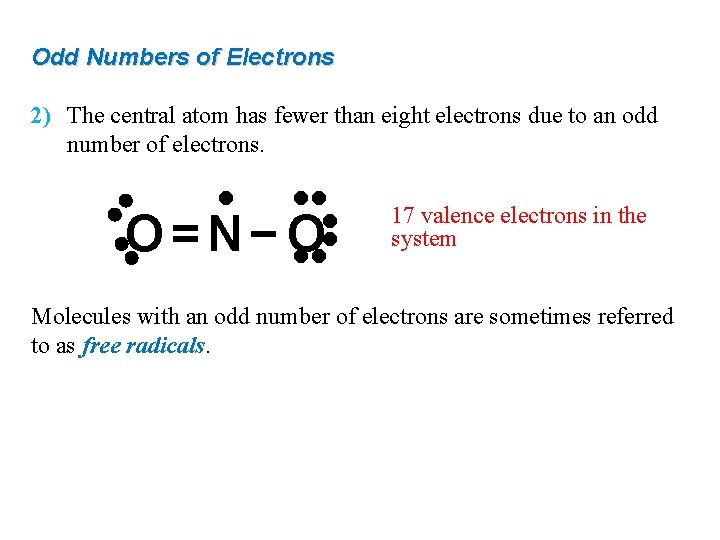
Odd Numbers of Electrons 2) The central atom has fewer than eight electrons due to an odd number of electrons. • • • • O=N−O 17 valence electrons in the system Molecules with an odd number of electrons are sometimes referred to as free radicals.

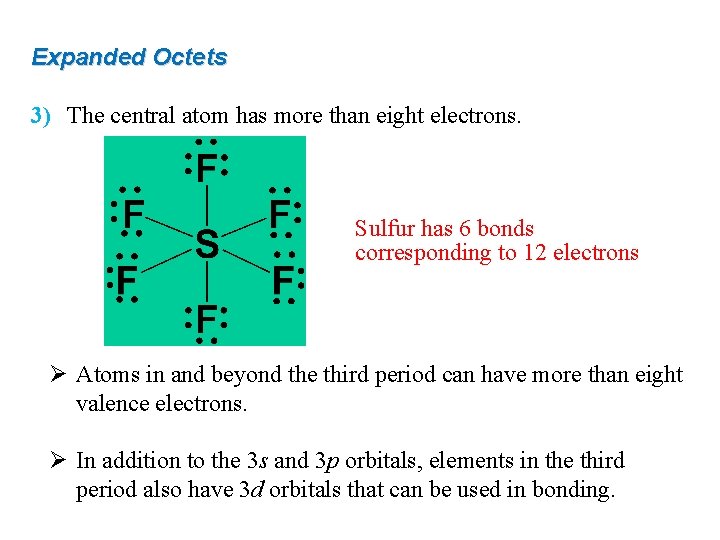
Expanded Octets 3) The central atom has more than eight electrons. Sulfur has 6 bonds corresponding to 12 electrons Ø Atoms in and beyond the third period can have more than eight valence electrons. Ø In addition to the 3 s and 3 p orbitals, elements in the third period also have 3 d orbitals that can be used in bonding.

Worked Example 6. 9 Draw the Lewis structure of boron triiodide (BI 3).

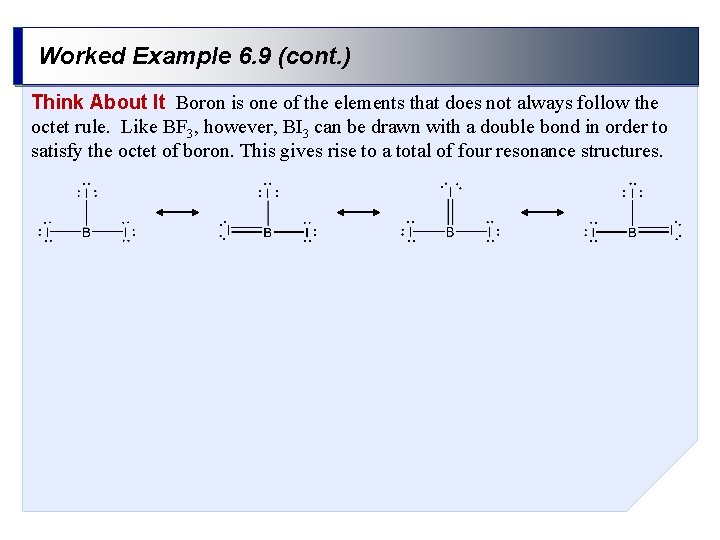
Worked Example 6. 9 (cont. ) Think About It Boron is one of the elements that does not always follow the octet rule. Like BF 3, however, BI 3 can be drawn with a double bond in order to satisfy the octet of boron. This gives rise to a total of four resonance structures.

Worked Example 6. 9 Draw the Lewis structure of chlorine dioxide (Cl. O 2).

Worked Example 6. 11 Draw two resonance structures for sulfurous acid (H 2 SO 3): one that obeys the octet rule for the central atom, and one that minimizes the formal charges. Determine the formal charges on each atom in both structures.

Study Guide for Sections 6. 4 -6. 6 DAY 12, Terms to know: Sections 6. 4 -6. 6: formal charge, resonance, free radical DAY 12, Specific outcomes and skills that may be tested on exam 2: Sections 6. 4 -6. 6 • Be able to calculate formal charges for atoms in Lewis structures • Be able to draw all resonance contributors for a given Lewis structure and explain how resonance stabilizes molecules • Be able to draw reasonable Lewis structures for molecules that are exceptions to the octet rule

Extra Practice Problems for Sections 6. 4 -6. 6 Complete these problems outside of class until you are confident you have learned the SKILLS in this section outlined on the study guide and we will review some of them next class period. 6. 29 6. 31 6. 35 6. 37 6. 39 6. 49 6. 55 6. 57 6. 59 6. 65 6. 73 6. 75 6. 77 6. 79

Prep for Day 13 Must Watch videos: https: //www. youtube. com/watch? v=nxeb. QZUVv. Tg (VSEPR, Tyler De. Witt) https: //www. youtube. com/watch? v=8 Tl_b. DWCAmo (VSEPR, Tyler De. Witt) http: //ps. uci. edu/content/chem-1 a-general-chemistry (UC-Irvine lecture 10, watch the first 22 minutes and from 40 minutes until the end) https: //www. youtube. com/watch? v=15 Nvvwu. Tr 5 E (bond polarity) Other helpful videos: http: //echem 1 a. cchem. berkeley. edu/modules/module-4/ (UC-Berkeley lesson 12) http: //ocw. mit. edu/courses/chemistry/5 -111 -principles-of-chemical-science-fall-2008/video-lectures/ (MIT lecture 13) Read Sections 7. 1 and 6. 2 (pages 196 -199)