Lewis Dot Structures Lewis Dot Structures Lewis dot





- Slides: 12

Lewis Dot Structures

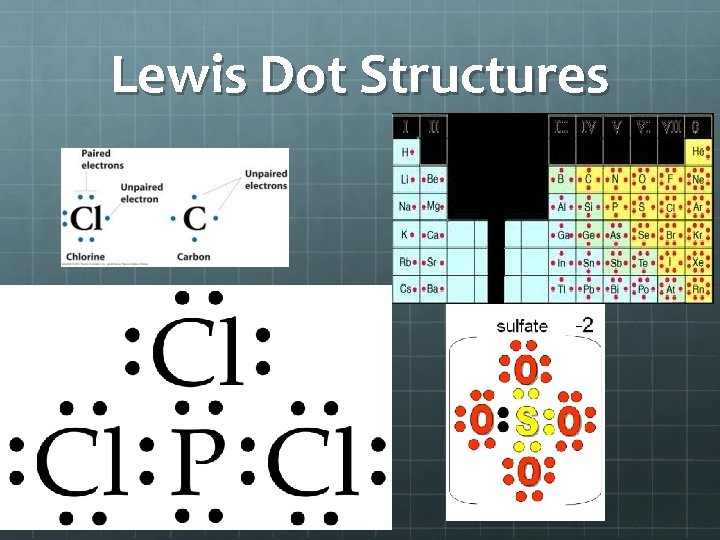
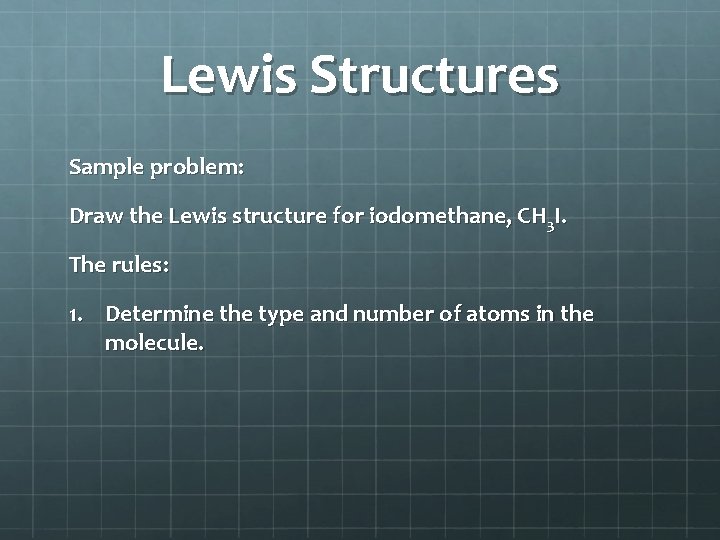
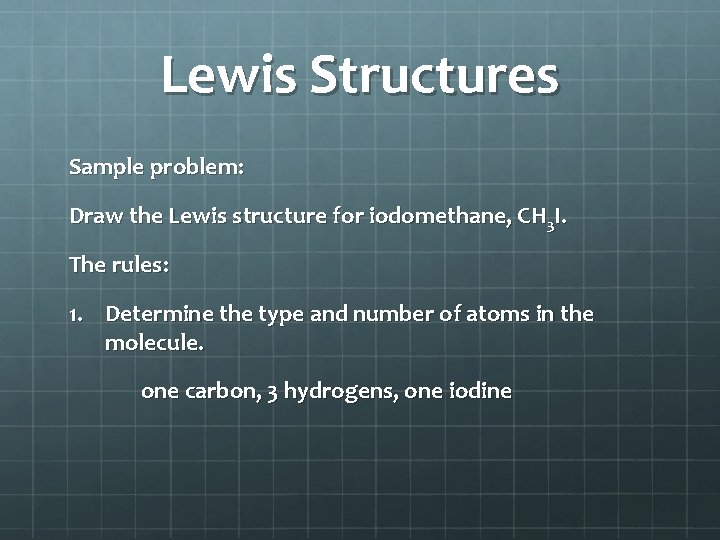
Lewis Dot Structures Lewis dot structures = Lewis structures = electron dot diagrams These represent formulas of atoms or molecules in which atomic symbols represent the nucleus of atoms and dots represents valence electrons. Dashes can represent shared electron pairs There are rules to drawing Lewis structures!!!

Lewis Structures Sample problem: Draw the Lewis structure for iodomethane, CH 3 I. The rules: 1. Determine the type and number of atoms in the molecule.

Lewis Structures Sample problem: Draw the Lewis structure for iodomethane, CH 3 I. The rules: 1. Determine the type and number of atoms in the molecule. one carbon, 3 hydrogens, one iodine

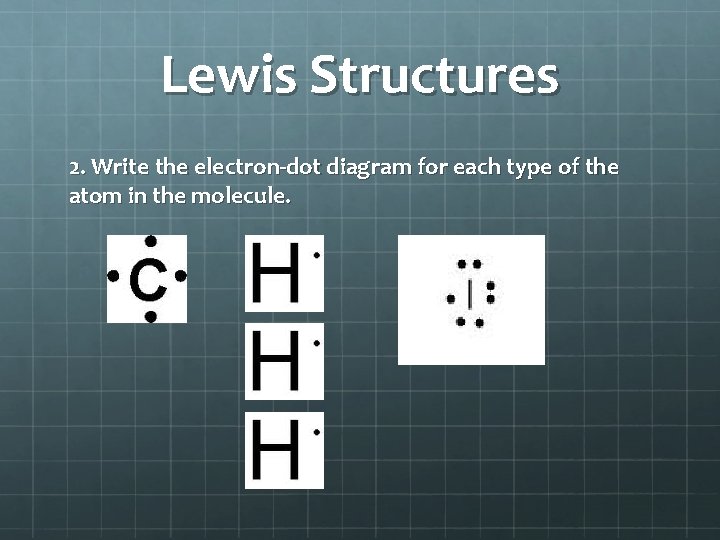
Lewis Structures 2. Write the electron-dot diagram for each type of the atom in the molecule.

Lewis Structures 2. Write the electron-dot diagram for each type of the atom in the molecule.

Lewis Structures 3. Determine the total number of valence electrons available in the atoms to be combined.

Lewis Structures 3. Determine the total number of valence electrons available in the atoms to be combined. Carbon = 4 electrons Hydrogen = 3 electrons (1 each) Iodine = 7 electrons 14 total electrons

Lewis Structures 4. Arrange the atoms to form a skeleton structure for the molecule. If carbon is present, it is central atom. Otherwise, the least electronegative atom is central (except for hydrogen, which is never central). Then connect the atoms by electron pairs.

Lewis Structures 5. Add unshared pairs of electrons to each nonmetal atom (except hydrogen) such that each is surrounded by 8 electrons.

Lewis Structures 6. Count the electrons in the structure to be sure that the number of valence electrons used equals the number available. Be sure the central atom and other atoms besides hydrogen have an octet (8 electrons)

Try it yourself! Draw the Lewis structure for ammonia, NH 3. Draw the Lewis structure for hydrogen sulfide, H 2 S. Draw the Lewis structure for silane, Si. H 4. Draw the Lewis structure for phosphorus trifluoride, PF 3.