Lewis Structures and Formal Charge Rules Governing Formal




- Slides: 11

Lewis Structures and Formal Charge

Rules Governing Formal Charge • Calculate Formal Charge • Add up the lone pair electrons on the atom, and half of the shared electrons around the atom (those in bonds) • Subtract the assigned electrons from the element’s valence electrons • The sum of the formal charges on all atoms in a given molecule or ion must equal the overall charge on the species • Molecules = zero • Ions = ionic charge • Which is the preferred Lewis Structure? • Formal charges closest to zero • Negative formal charges on the most electronegative atoms

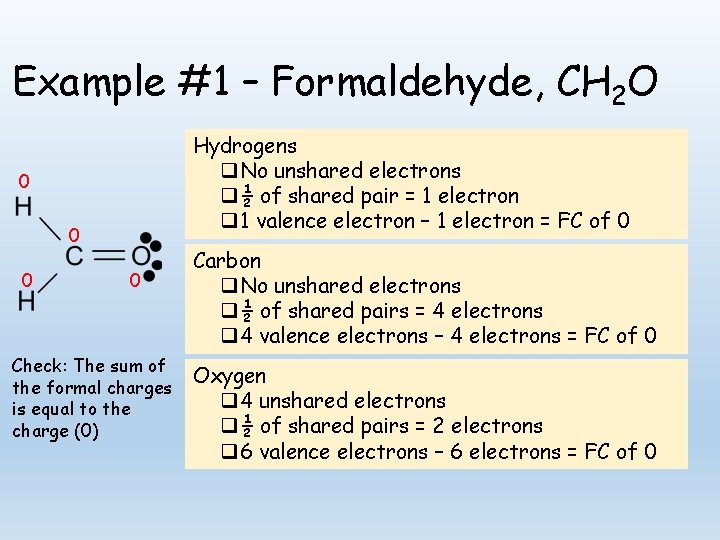
Example #1 – Formaldehyde, CH 2 O Hydrogens q No unshared electrons q ½ of shared pair = 1 electron q 1 valence electron – 1 electron = FC of 0 0 0 Check: The sum of the formal charges is equal to the charge (0) Carbon q No unshared electrons q ½ of shared pairs = 4 electrons q 4 valence electrons – 4 electrons = FC of 0 Oxygen q 4 unshared electrons q ½ of shared pairs = 2 electrons q 6 valence electrons – 6 electrons = FC of 0

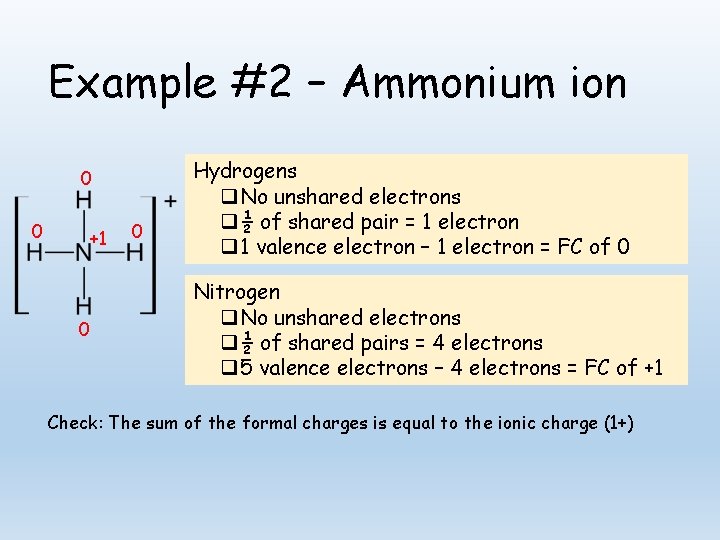
Example #2 – Ammonium ion 0 0 +1 0 0 Hydrogens q No unshared electrons q ½ of shared pair = 1 electron q 1 valence electron – 1 electron = FC of 0 Nitrogen q No unshared electrons q ½ of shared pairs = 4 electrons q 5 valence electrons – 4 electrons = FC of +1 Check: The sum of the formal charges is equal to the ionic charge (1+)

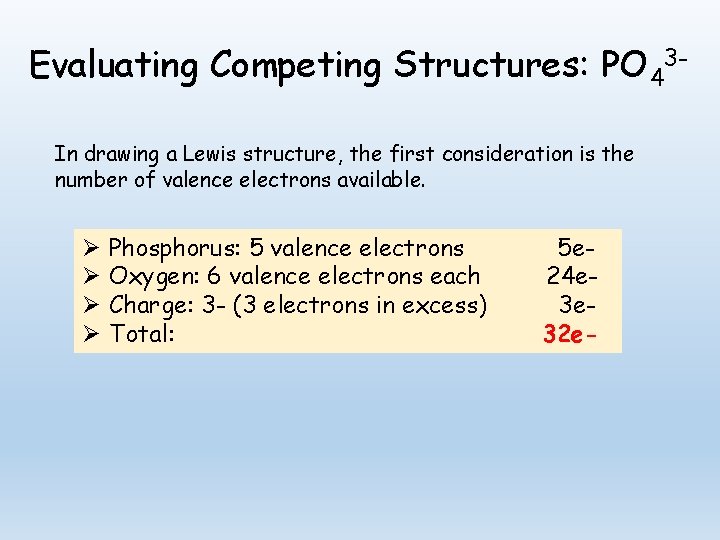
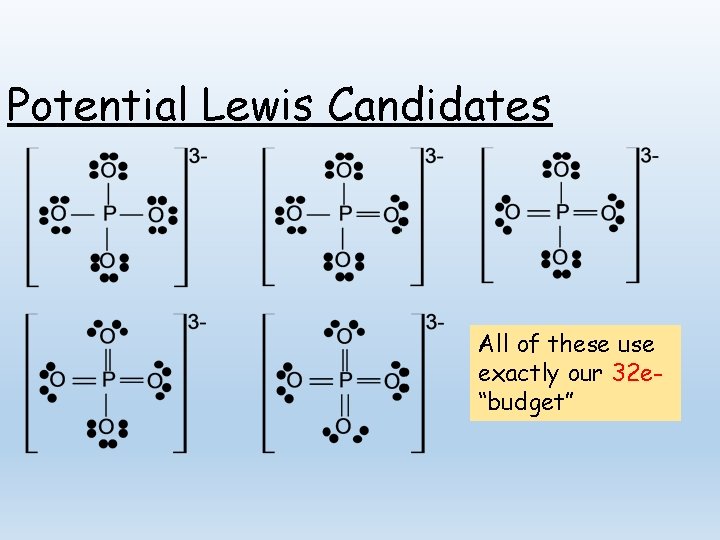
Evaluating Competing Structures: PO 43 In drawing a Lewis structure, the first consideration is the number of valence electrons available. Ø Ø Phosphorus: 5 valence electrons Oxygen: 6 valence electrons each Charge: 3 - (3 electrons in excess) Total: 5 e 24 e 3 e 32 e-

Potential Lewis Candidates All of these use exactly our 32 e“budget”

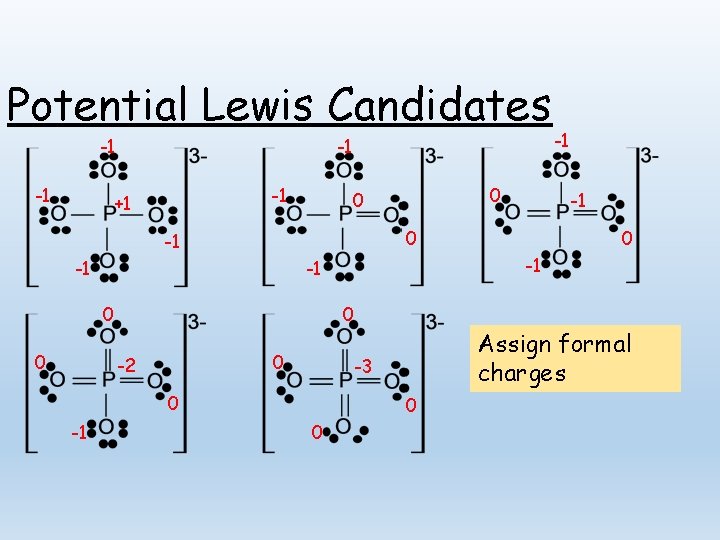
Potential Lewis Candidates -1 -1 +1 0 0 0 -1 Assign formal charges -3 0 0 -1 -1 -1 -2 -1 0 0 0 -1

Evaluate Using Formal Charges 0 0 -3 0 0 Oxygen is more electronegative than phosphorus. Negative formal charge should be on oxygen.

Evaluate Using Formal Charges 0 0 -2 0 -1 Oxygen is more electronegative than phosphorus. Negative formal charge should be on oxygen.

Evaluate Using Formal Charges -1 0 -1 Oxygen is more electronegative than phosphorus. Negative formal charge should be on oxygen.

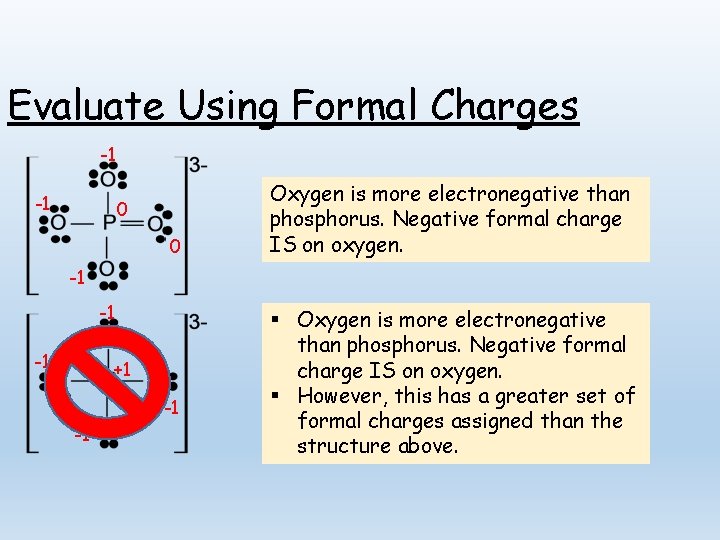
Evaluate Using Formal Charges -1 -1 0 0 Oxygen is more electronegative than phosphorus. Negative formal charge IS on oxygen. -1 -1 -1 +1 -1 -1 § Oxygen is more electronegative than phosphorus. Negative formal charge IS on oxygen. § However, this has a greater set of formal charges assigned than the structure above.