Resonance and Formal Charge 1 Resonance and Formal















- Slides: 32

Resonance and Formal Charge 1

Resonance and Formal Charge: At the conclusion of our time together, you should be able to: 1. Define resonance 2. Determine resonance structures for a molecule 3. Calculate the formal charge for an atom 4. Determine the resonance structure that contributes the most to a compound by using formal charge 2

Redneck Jet Skiing 3

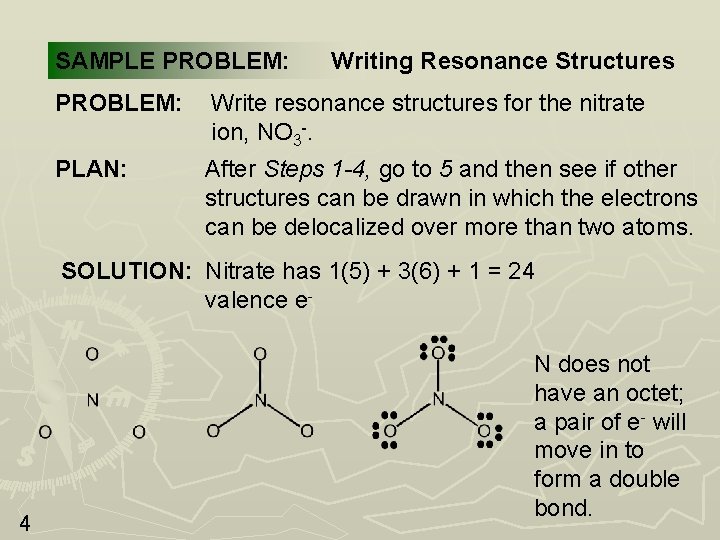
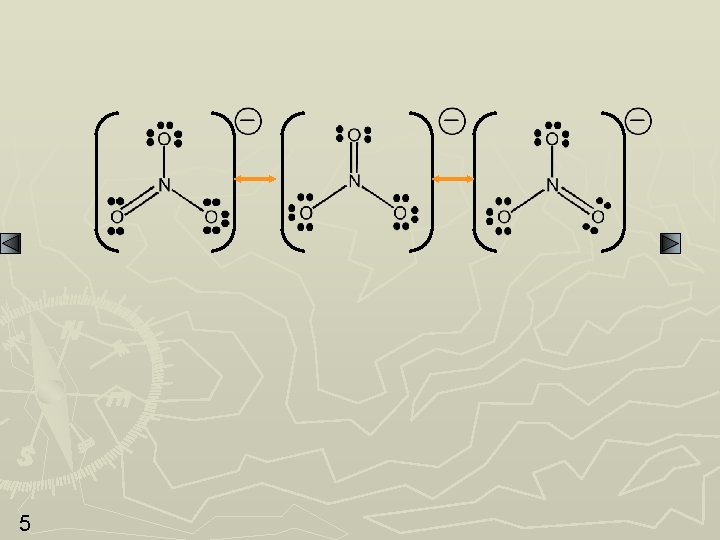
SAMPLE PROBLEM: Writing Resonance Structures PROBLEM: Write resonance structures for the nitrate ion, NO 3 -. PLAN: After Steps 1 -4, go to 5 and then see if other structures can be drawn in which the electrons can be delocalized over more than two atoms. SOLUTION: Nitrate has 1(5) + 3(6) + 1 = 24 valence e- 4 N does not have an octet; a pair of e- will move in to form a double bond.

5

Four criteria for choosing the more important resonance structure: 1. Smaller formal charges (either positive or negative) are preferable to larger charges; 2. A more negative formal charge should exist on an atom with a larger EN value. 3. Get unlike charges as close together as possible 6 4. Avoid like charges (+ + or - - ) on adjacent atoms

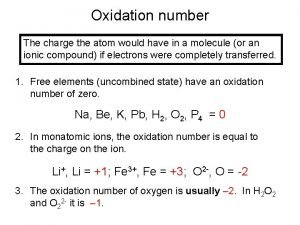
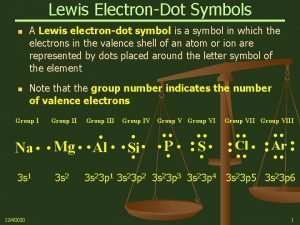
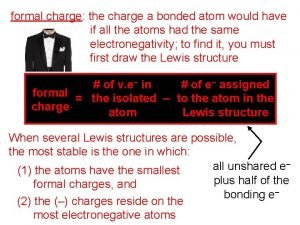
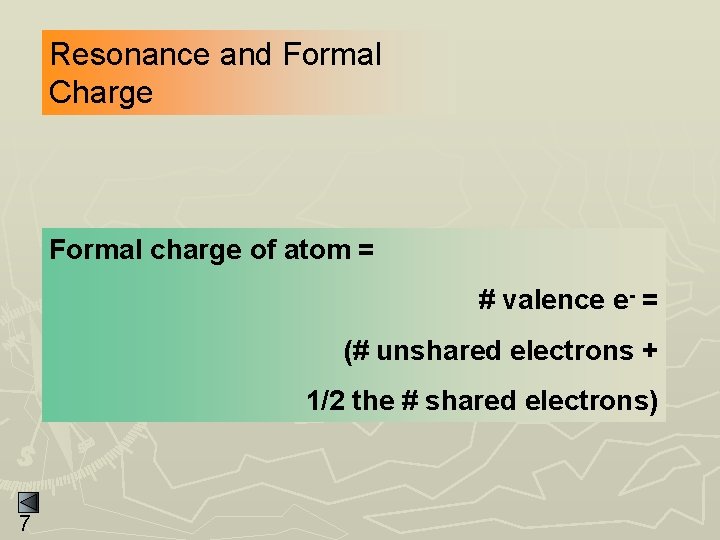
Resonance and Formal Charge Formal charge of atom = # valence e- = (# unshared electrons + 1/2 the # shared electrons) 7

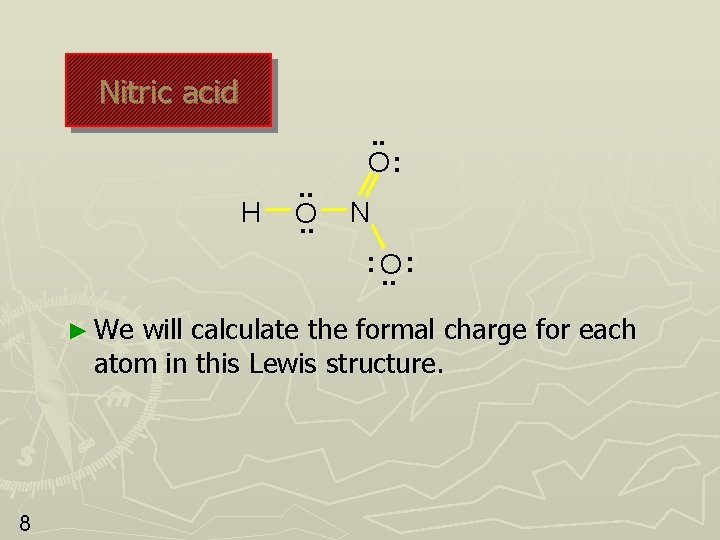
Nitric acid H . . O: N : O. . : ► We will calculate the formal charge for each atom in this Lewis structure. 8

9

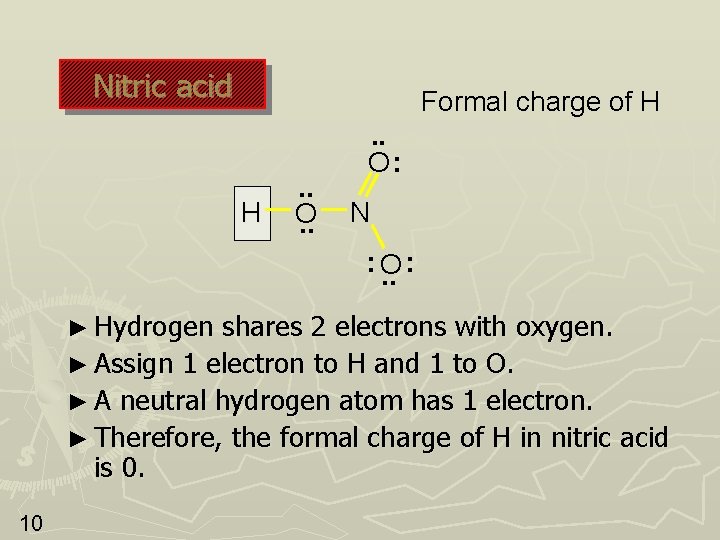
Nitric acid Formal charge of H H . . O: N : O. . : ► Hydrogen shares 2 electrons with oxygen. ► Assign 1 electron to H and 1 to O. ► A neutral hydrogen atom has 1 electron. ► Therefore, the formal charge of H in nitric acid is 0. 10

Nitric acid Formal charge of O. . H O. . O: N : O. . : ► Oxygen 11 has 4 electrons in covalent bonds. ► Assign 2 of these 4 electrons to O. ► Oxygen has 2 unshared pairs. Assign all 4 of these electrons to O. ► Therefore, the total number of electrons assigned to O is 2 + 4 = 6.

Nitric acid Formal charge of O. . H O. . O: N : O. . : ► Electron count of O is 6. ► A neutral oxygen has 6 electrons. ► Therefore, the formal charge of oxygen is 0. 12

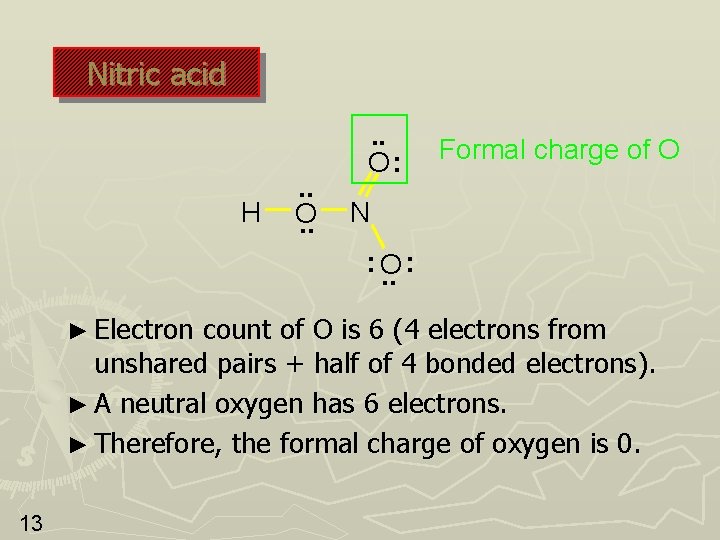
Nitric acid H . . O: Formal charge of O N : O. . : ► Electron count of O is 6 (4 electrons from unshared pairs + half of 4 bonded electrons). ► A neutral oxygen has 6 electrons. ► Therefore, the formal charge of oxygen is 0. 13

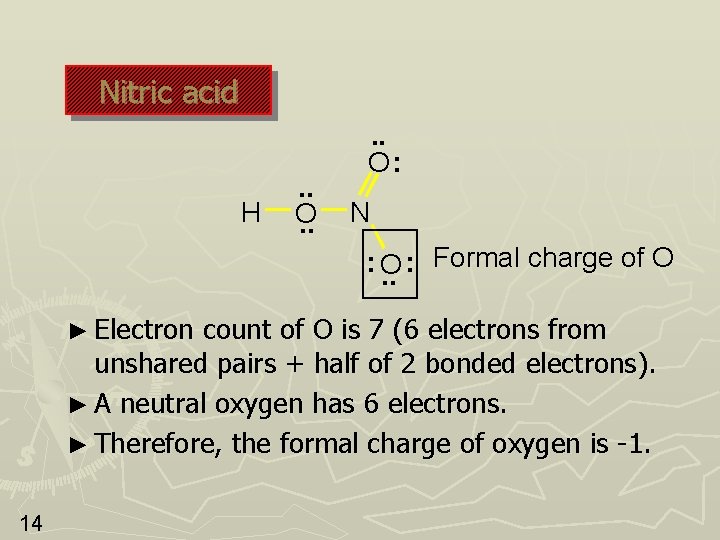
Nitric acid H . . O: N Formal charge of O : : O. . ► Electron count of O is 7 (6 electrons from unshared pairs + half of 2 bonded electrons). ► A neutral oxygen has 6 electrons. ► Therefore, the formal charge of oxygen is -1. 14

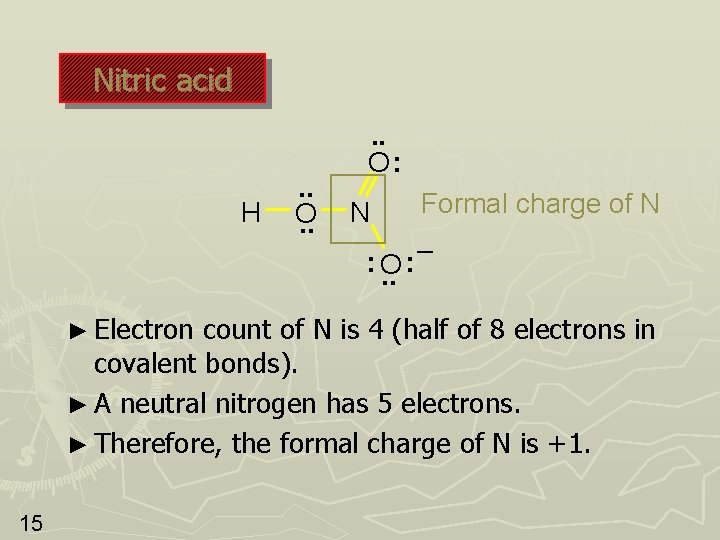
Nitric acid H . . O: N : O. . ► Electron Formal charge of N – : count of N is 4 (half of 8 electrons in covalent bonds). ► A neutral nitrogen has 5 electrons. ► Therefore, the formal charge of N is +1. 15

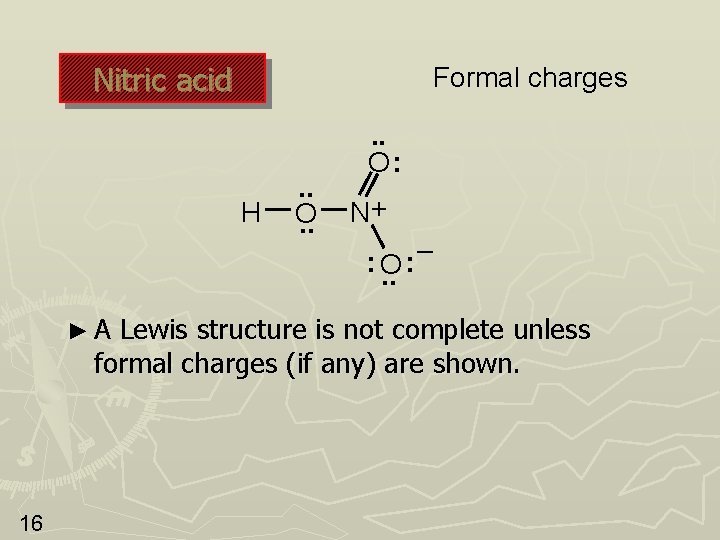
Nitric acid Formal charges H . . O: N+ : O. . ►A – : Lewis structure is not complete unless formal charges (if any) are shown. 16

Sure!! And I suppose the Pope is Jewish? ? ? 17

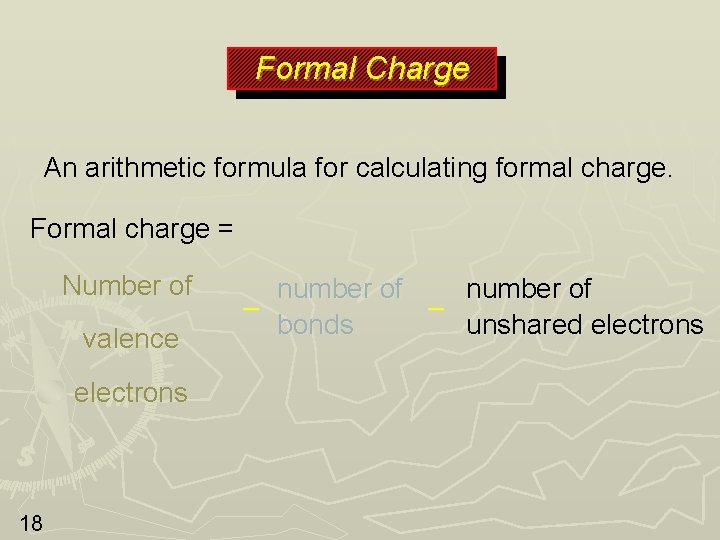
Formal Charge An arithmetic formula for calculating formal charge. Formal charge = Number of valence electrons 18 number of – – bonds unshared electrons

"Electron Counts" and Formal Charges in NH 4+ and BF 4 - 1 H H 4 19 N H + H . . : F: . . –. . : . . F B. . F: : . . F: 7 4

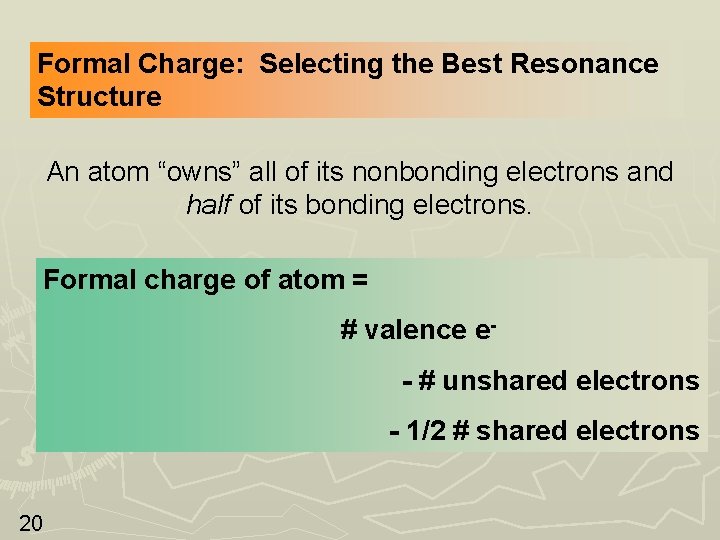
Formal Charge: Selecting the Best Resonance Structure An atom “owns” all of its nonbonding electrons and half of its bonding electrons. Formal charge of atom = # valence e- # unshared electrons - 1/2 # shared electrons 20

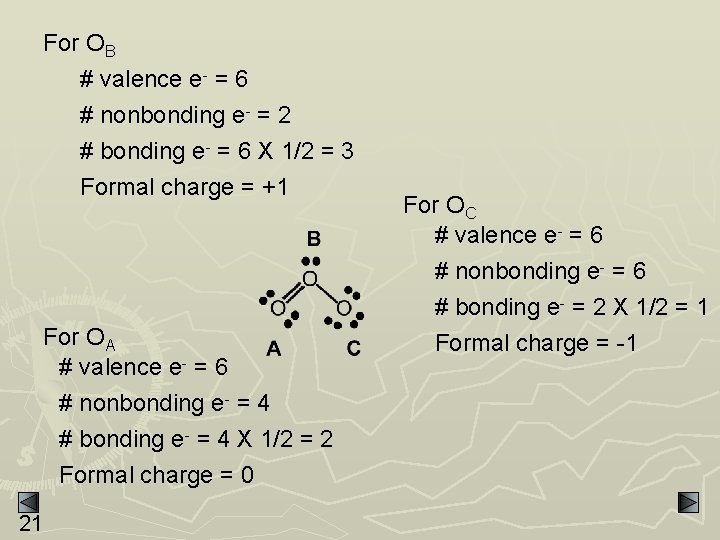
For OB # valence e- = 6 # nonbonding e- = 2 # bonding e- = 6 X 1/2 = 3 Formal charge = +1 For OA # valence e- = 6 # nonbonding e- = 4 # bonding e- = 4 X 1/2 = 2 Formal charge = 0 21 For OC # valence e- = 6 # nonbonding e- = 6 # bonding e- = 2 X 1/2 = 1 Formal charge = -1

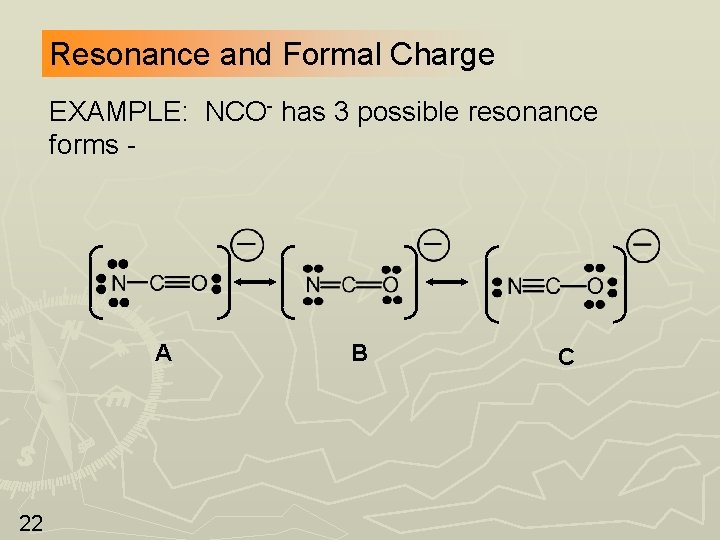
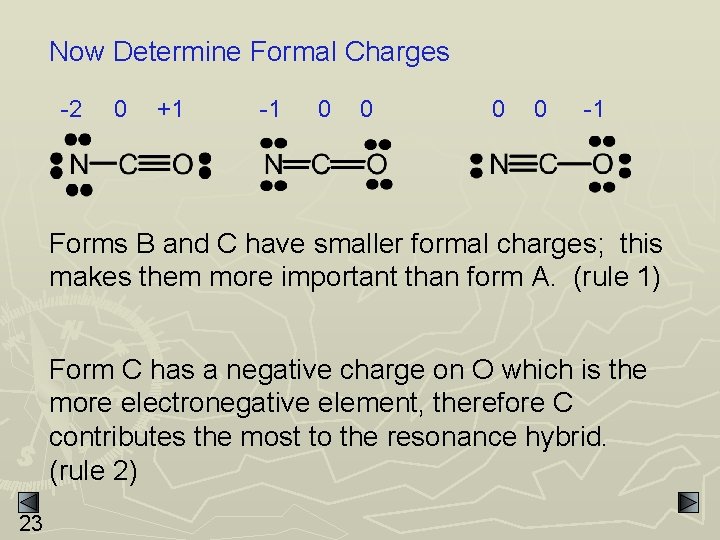
Resonance and Formal Charge EXAMPLE: NCO- has 3 possible resonance forms - A 22 B C

Now Determine Formal Charges -2 0 +1 -1 0 0 -1 Forms B and C have smaller formal charges; this makes them more important than form A. (rule 1) Form C has a negative charge on O which is the more electronegative element, therefore C contributes the most to the resonance hybrid. (rule 2) 23

Redneck NASCAR Racing 24

Exceptions to the Octet Rule 25

Exceptions to the Octet Rule ► 4 b. Expanded Octets – only on period 3 and higher § Expanded octets form when an atom can decrease (or maintain at 0) it’s formal charge § Ex: SF 6, PCl 5, SO 2, SO 3, SO 4 ► 5 a. Electron deficient – have fewer than 8 § Ex: Be. Cl 2, BF 3 § may attain an octet by coordinate covalent bond ► Odd number of electrons – aka free radicals § Ex: NO 2 § May attain an octet by pairing with another free radical 26

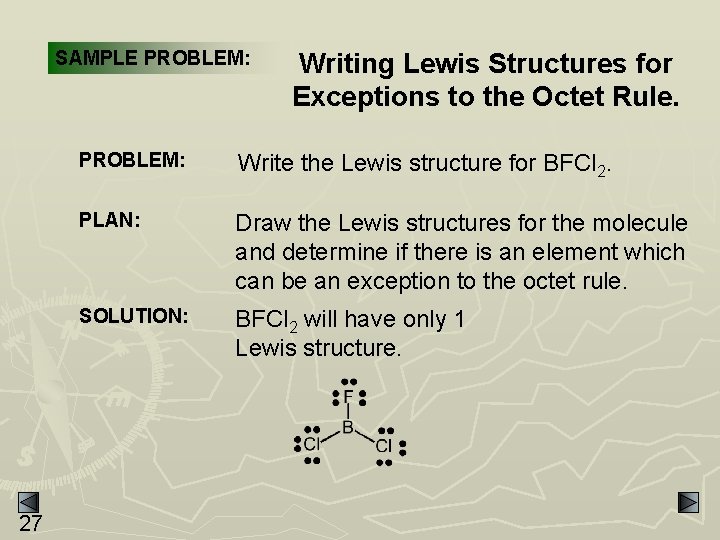
SAMPLE PROBLEM: 27 Writing Lewis Structures for Exceptions to the Octet Rule. PROBLEM: Write the Lewis structure for BFCl 2. PLAN: Draw the Lewis structures for the molecule and determine if there is an element which can be an exception to the octet rule. SOLUTION: BFCl 2 will have only 1 Lewis structure.

Resonance and Formal Charge: Let’s see if you can: 1. Define resonance 2. Determine resonance structures for a molecule 3. Calculate the formal charge for an atom 4. Determine the resonance structure that contributes the most to a compound by using formal charge 28

Your Turn Now determine the formal charges and best structure for the 2 examples at the bottom of page 11. 29

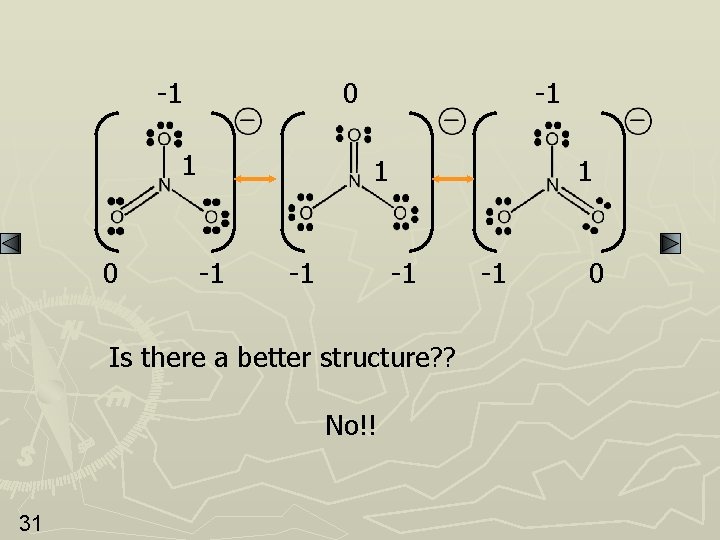
Your Turn Now determine the formal charges and best structure for the middle example on page 12. 30

-1 0 -1 1 -1 Is there a better structure? ? No!! 31 -1 0

The base is under assault!!! 32
 Formal charge nco-
Formal charge nco- Formal charges
Formal charges Formal charge formula
Formal charge formula Difference between charge and electric charge
Difference between charge and electric charge Difference between charge and electric charge
Difference between charge and electric charge Hnc lewis dot structure
Hnc lewis dot structure Oxidation number vs formal charge
Oxidation number vs formal charge Sf4cl lewis structure
Sf4cl lewis structure Calculating formal charge
Calculating formal charge Lewis structure for hcn
Lewis structure for hcn How to calculate formal charge
How to calculate formal charge Calculating formal charge
Calculating formal charge Nitric acid formal charge
Nitric acid formal charge Which atom in hcn will bear a formal charge?
Which atom in hcn will bear a formal charge? 021131141
021131141 Formal charge
Formal charge Oncl formal charge
Oncl formal charge Hofo lewis structure
Hofo lewis structure Sound intensity and resonance
Sound intensity and resonance Forced vibration and resonance
Forced vibration and resonance Half open pipe
Half open pipe Forced vibration and resonance
Forced vibration and resonance Brand resonance and the brand value chain
Brand resonance and the brand value chain The brand value chain
The brand value chain Delocalization and resonance
Delocalization and resonance Vocal resonance
Vocal resonance Percussion notes in lungs
Percussion notes in lungs Percussion tympanic
Percussion tympanic Lewis structure for chocl
Lewis structure for chocl Resonance brand
Resonance brand 3 point perspective stairs
3 point perspective stairs Resonance in nmr
Resonance in nmr S2o lewis structure resonance
S2o lewis structure resonance