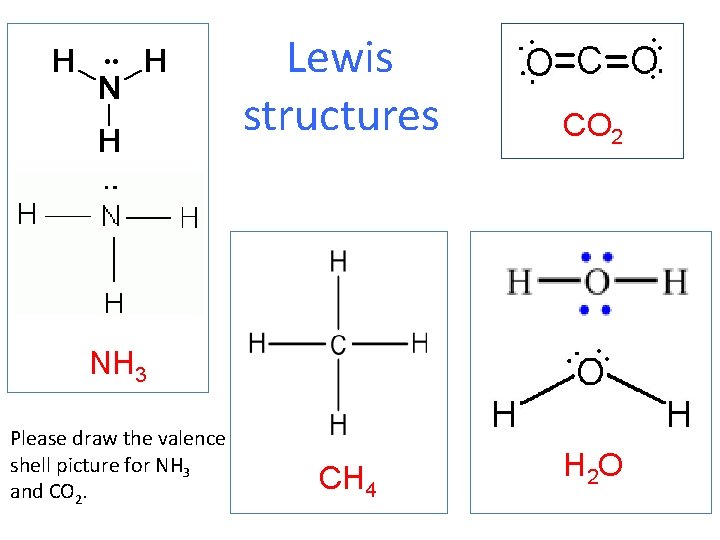
Lewis structures and VSEPR Lewis structures CO 2
















- Slides: 41

Lewis structures and VSEPR

Lewis structures CO 2 CH 4 H 2 O NH 3 Please draw the valence shell picture for NH 3 and CO 2.

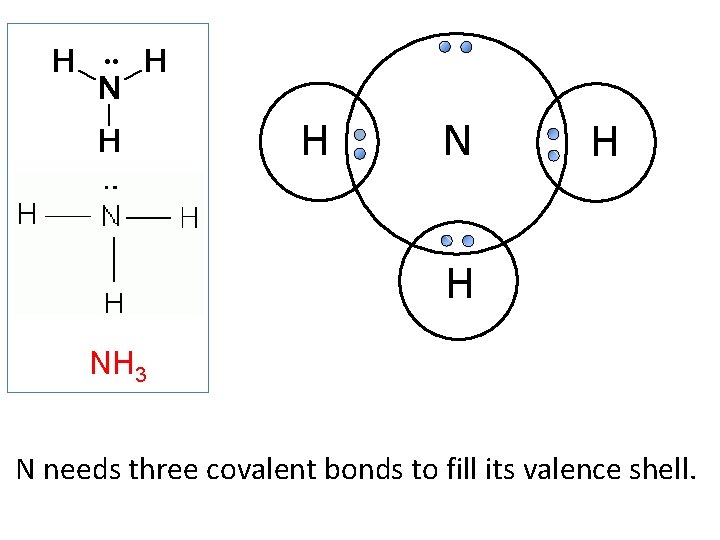
H N H H NH 3 N needs three covalent bonds to fill its valence shell.

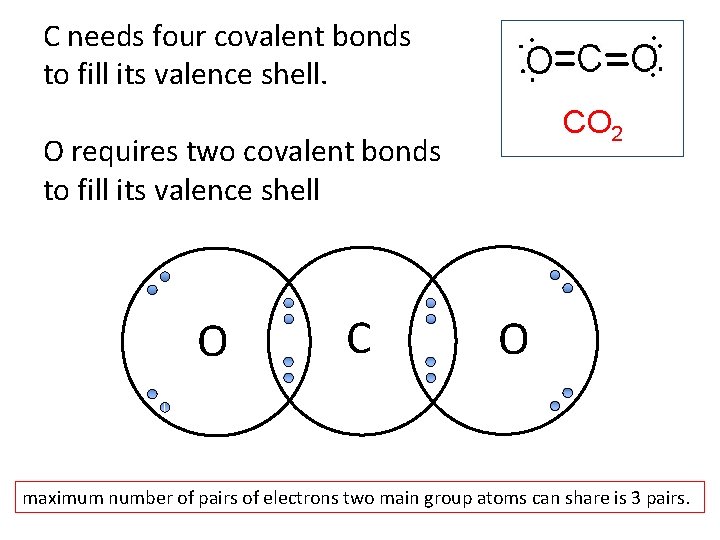
C needs four covalent bonds to fill its valence shell. CO 2 O requires two covalent bonds to fill its valence shell O C O maximum number of pairs of electrons two main group atoms can share is 3 pairs.

Lewis structures show the bonding. Lewis structures do not show the molecular shape.

Valence Shell Electron Pair Repulsion or VSEPR model gives molecular shape.

L. S. Bartell J. Chem. Educ. , 1968, 45 (12), p 754 Bonds formation, not electron pair repulsion, is responsible for molecular geometries.

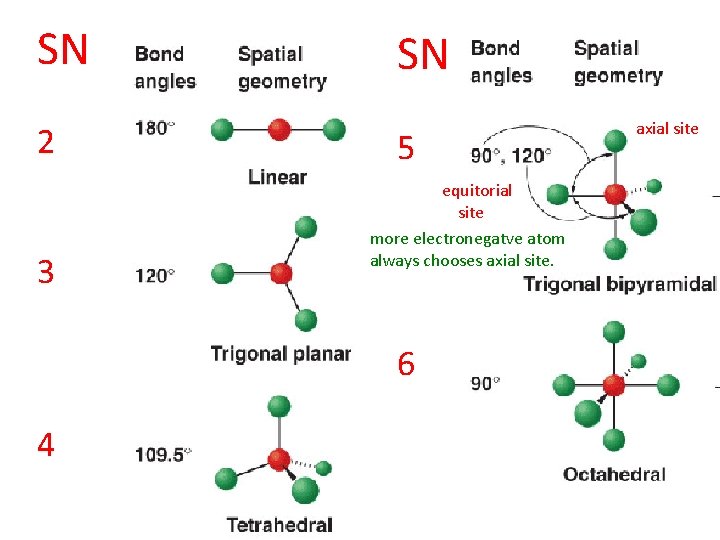
VSEPR: (1) Determine steric number (SN) (2) Use SN to predict molecular shape

SN 2 3 SN 5 equitorial site more electronegatve atom always chooses axial site. 6 4 axial site

two methods to determine SN for VSEPR 1) central atom method 2) Lewis structure method

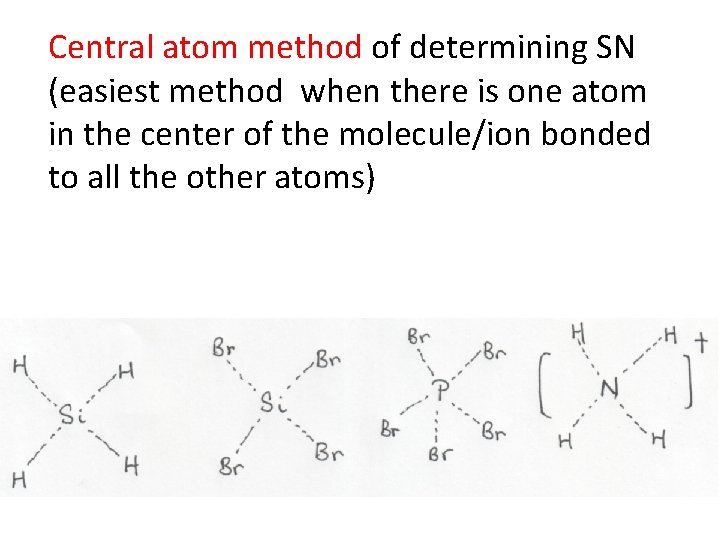
Central atom method of determining SN (easiest method when there is one atom in the center of the molecule/ion bonded to all the other atoms)

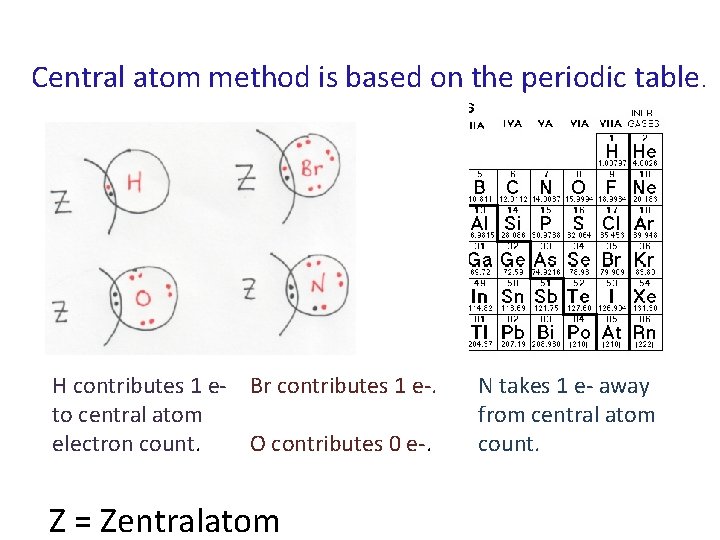
Central atom method is based on the periodic table. H contributes 1 e- Br contributes 1 e-. to central atom electron count. O contributes 0 e-. Z = Zentralatom N takes 1 e- away from central atom count.

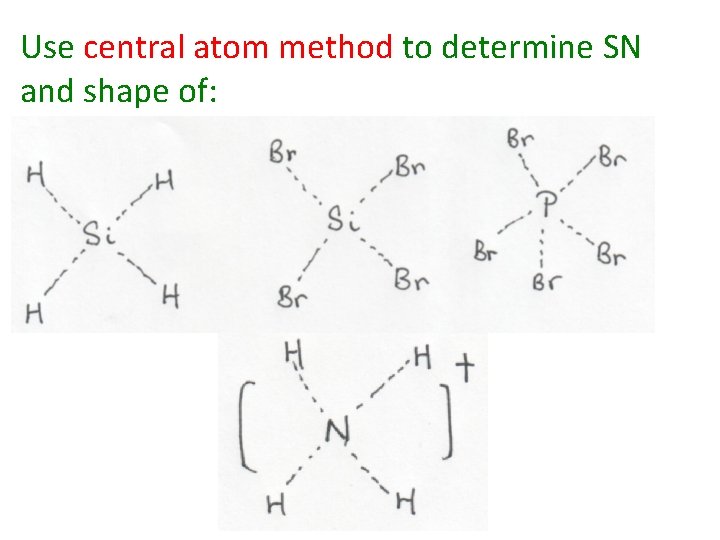
Use central atom method to determine SN and shape of:

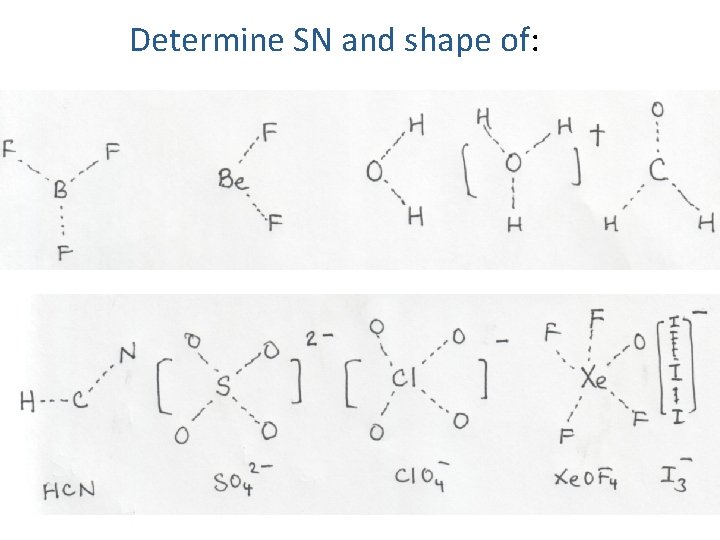
Determine SN and shape of:

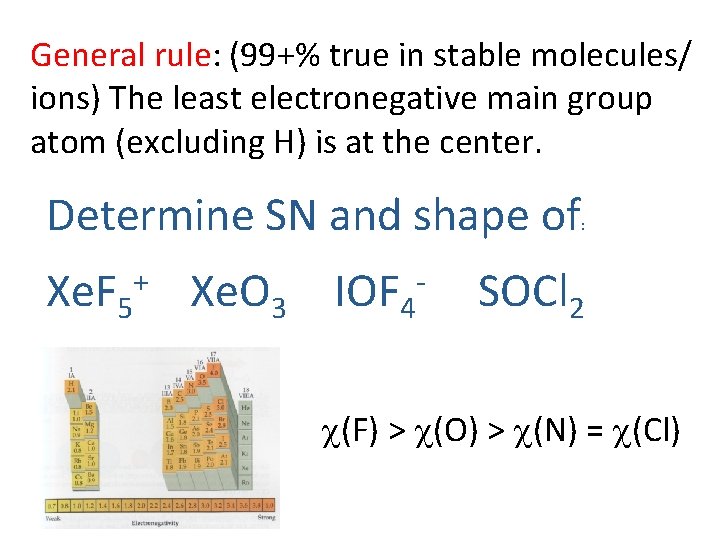
General rule: (99+% true in stable molecules/ ions) The least electronegative main group atom (excluding H) is at the center. Determine SN and shape of Xe. F 5+ Xe. O 3 IOF 4 - : SOCl 2 c(F) > c(O) > c(N) = c(Cl)

The remarkable molecule OFCl

two methods to determine SN for VSEPR 1) central atom method 2) Lewis structure method

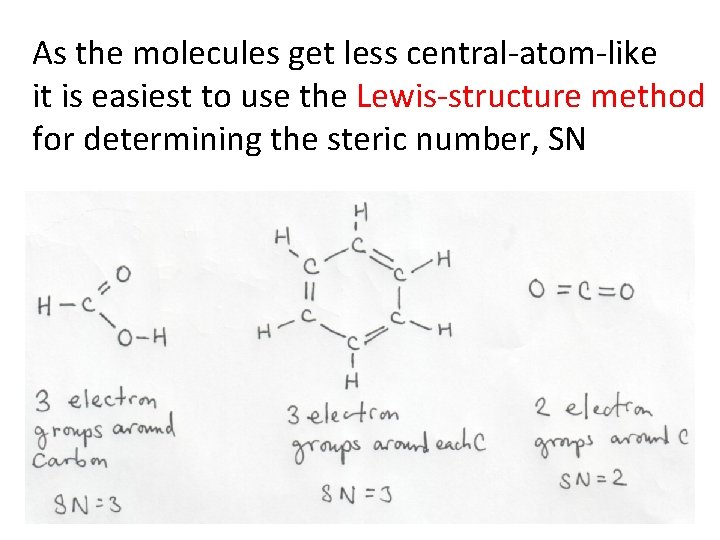
As the molecules get less central-atom-like it is easiest to use the Lewis-structure method for determining the steric number, SN

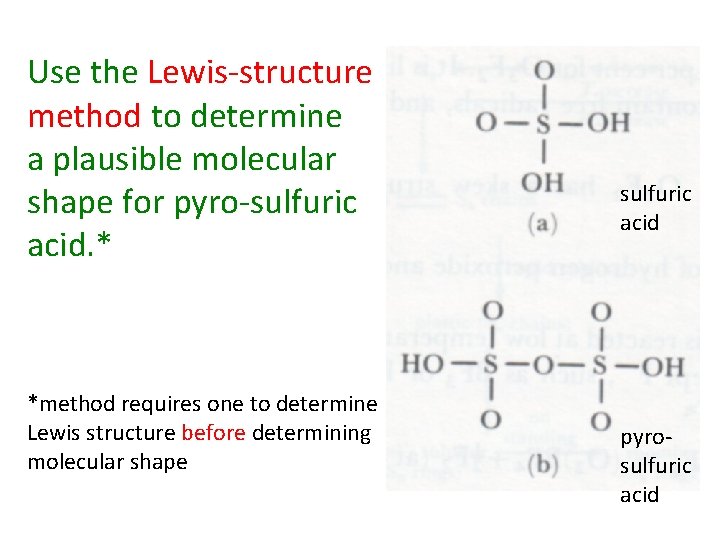
Use the Lewis-structure method to determine a plausible molecular shape for pyro-sulfuric acid. * *method requires one to determine Lewis structure before determining molecular shape sulfuric acid pyrosulfuric acid

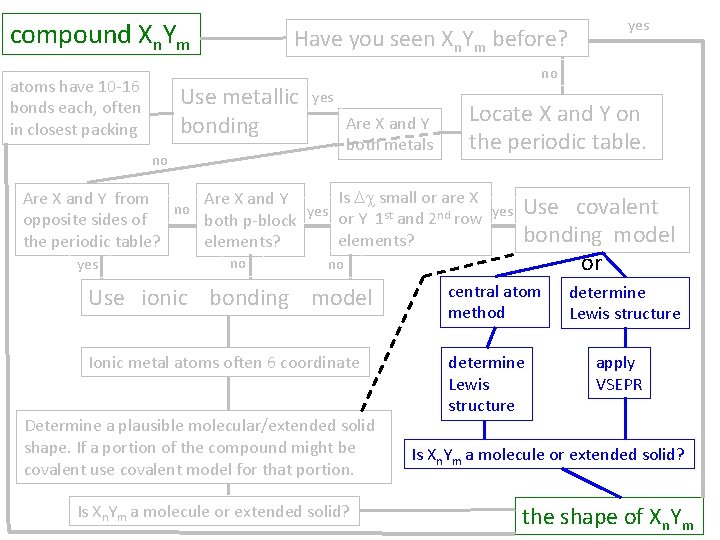
compound Xn. Ym atoms have 10 -16 bonds each, often in closest packing Have you seen Xn. Ym before? Use metallic bonding no yes Are X and Y both metals no Locate X and Y on the periodic table. Is Dc small or are X Are X and Y from Are X and Y no yes or Y 1 st and 2 nd row yes opposite sides of both p-block elements? the periodic table? elements? yes no Use covalent bonding model or Use ionic bonding model central atom method Ionic metal atoms often 6 coordinate determine Lewis structure Determine a plausible molecular/extended solid shape. If a portion of the compound might be covalent use covalent model for that portion. Is Xn. Ym a molecule or extended solid? determine Lewis structure apply VSEPR Is Xn. Ym a molecule or extended solid? the shape of Xn. Ym

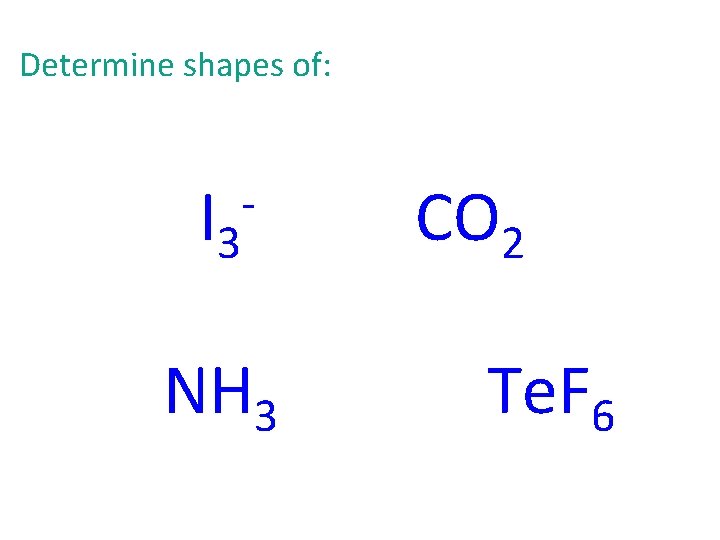
Determine shapes of: I 3 - NH 3 CO 2 Te. F 6

formal charge

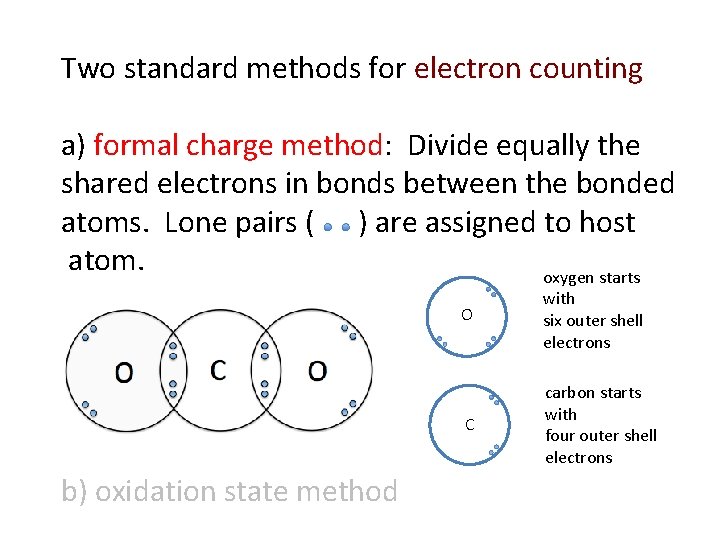
Two standard methods for electron counting a) formal charge method: Divide equally the shared electrons in bonds between the bonded atoms. Lone pairs ( ) are assigned to host atom. oxygen starts b) oxidation state method O with six outer shell electrons C carbon starts with four outer shell electrons

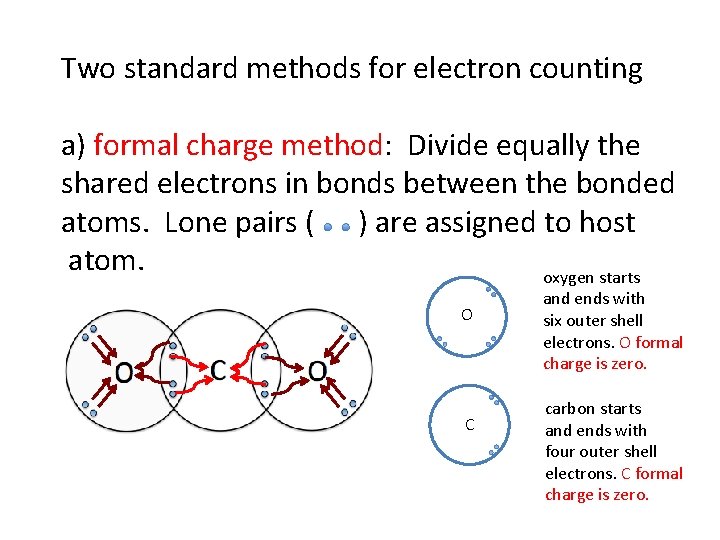
Two standard methods for electron counting a) formal charge method: Divide equally the shared electrons in bonds between the bonded atoms. Lone pairs ( ) are assigned to host atom. oxygen starts O C and ends with six outer shell electrons. O formal charge is zero. carbon starts and ends with four outer shell electrons. C formal charge is zero.

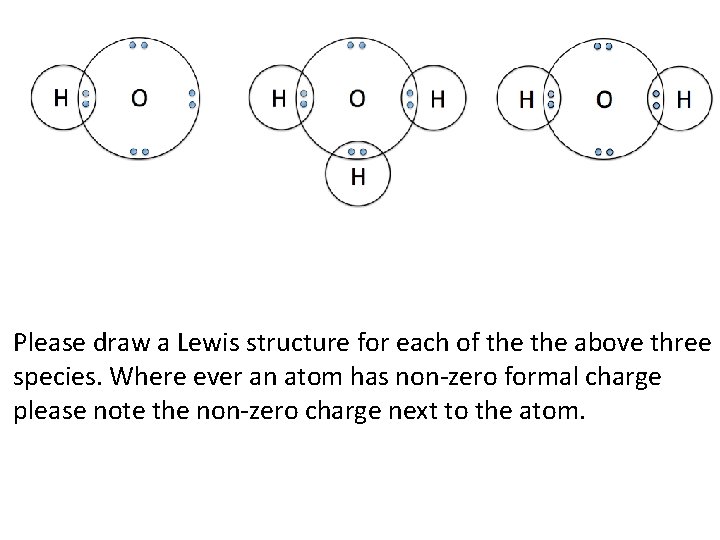
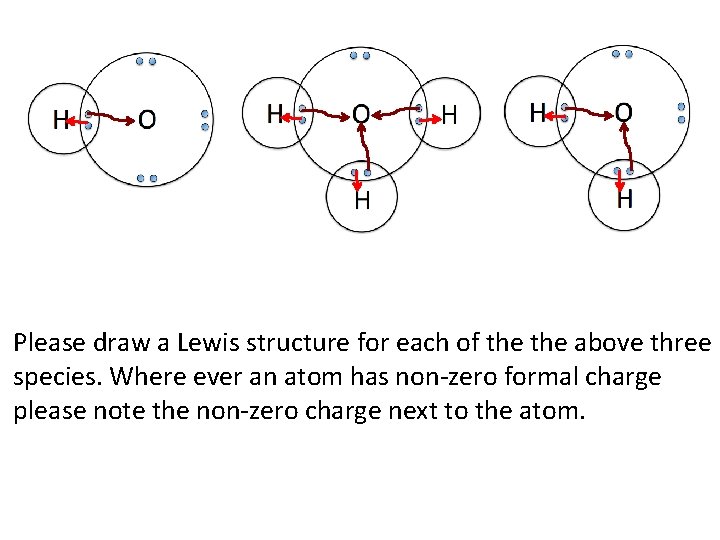
Please draw a Lewis structure for each of the above three species. Where ever an atom has non-zero formal charge please note the non-zero charge next to the atom.

Please draw a Lewis structure for each of the above three species. Where ever an atom has non-zero formal charge please note the non-zero charge next to the atom.

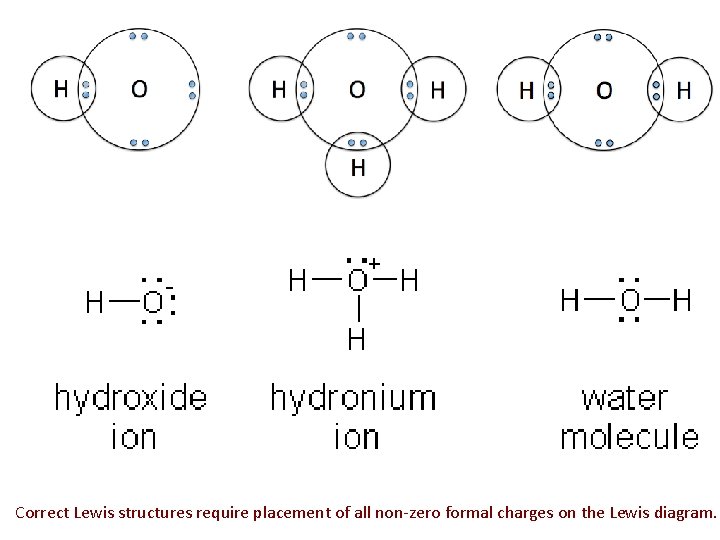
Correct Lewis structures require placement of all non-zero formal charges on the Lewis diagram.

octet rule

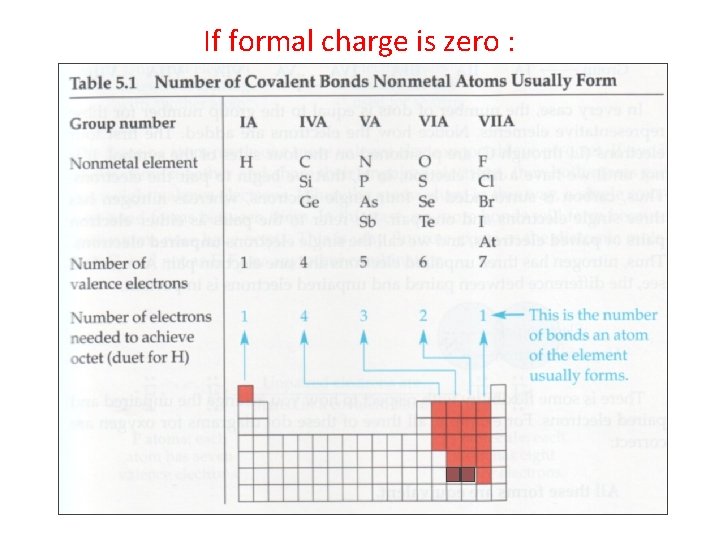
If formal charge is zero :

If formal charge is +1 then atom behaves as if it is an atom one column to the left. If formal charge is -1 then atom behaves as if it is an atom one column to the right. and so forth.

assuming atom has eight valence shell electrons.

assuming atom has eight outer shell electrons.

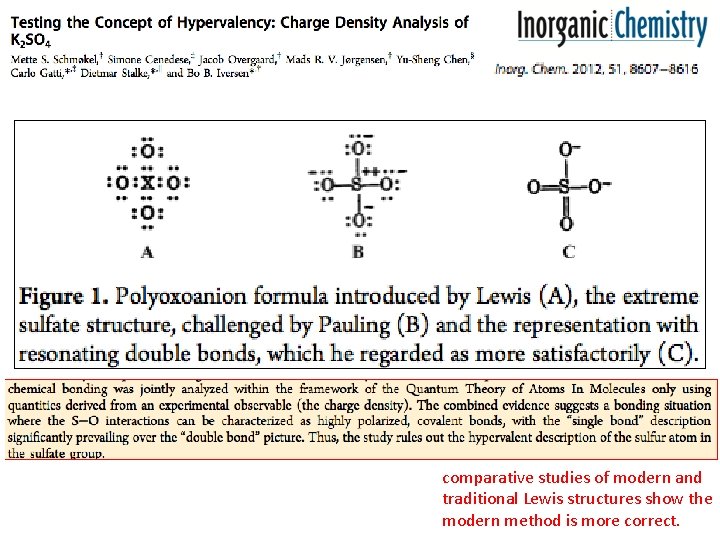
modern and traditional Lewis structures

deducing modern Lewis structures 1. if at all possible, all main group atoms (besides H) have an outer (valence) shell with eight electrons. Hydrogen just needs two. This is the octet rule. Outer shell electrons are called valence electrons. 2. having done step 1, make formal charges as small as possible. 3. no more than 3 bonds (multiple bonds) allowed between two main group atoms. One of the two atoms in this multiple bond is typically a second row element. it's a puzzle and needs to be solved like a puzzle

draw Lewis structures for all the ions below:

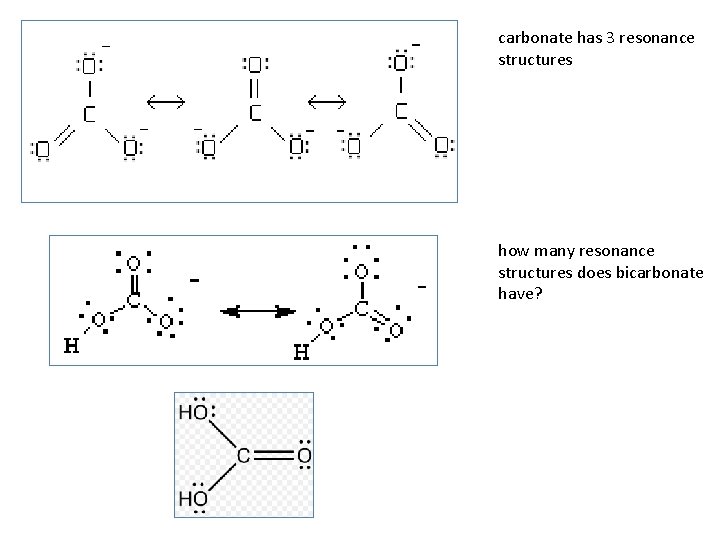
carbonate has 3 resonance structures how many resonance structures does bicarbonate have?

draw Lewis structures for all the ions to the left:

deducing traditional Lewis structures 1. make formal charges as small as possible. 2. second row main group elements almost always obey the octet rule. 3. other rows obey octet rule if it doesn't interfere with rules 1 and 2. 4. multiple bonds: allowed to make up to 3 bonds between a pair of main group atoms. One of the two atoms in multiple bond is typically a second row element. it's a puzzle and needs to be solved like a puzzle

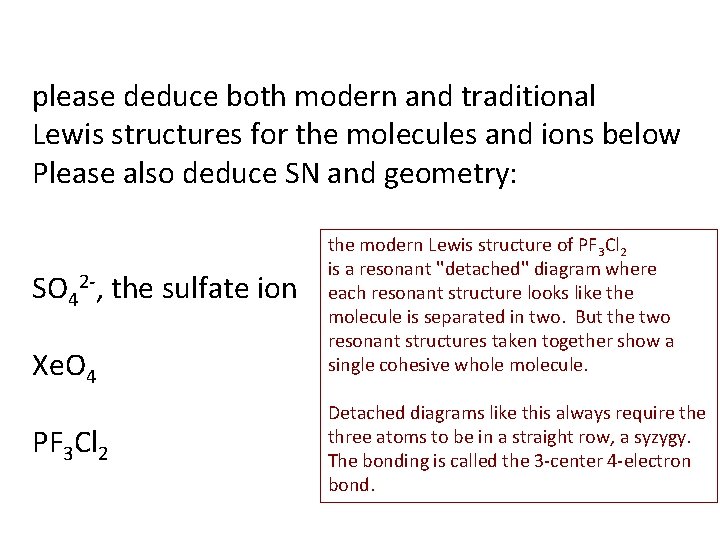
please deduce both modern and traditional Lewis structures for the molecules and ions below Please also deduce SN and geometry: SO 42 -, the sulfate ion Xe. O 4 PF 3 Cl 2 the modern Lewis structure of PF 3 Cl 2 is a resonant ''detached'' diagram where each resonant structure looks like the molecule is separated in two. But the two resonant structures taken together show a single cohesive whole molecule. Detached diagrams like this always require three atoms to be in a straight row, a syzygy. The bonding is called the 3 -center 4 -electron bond.

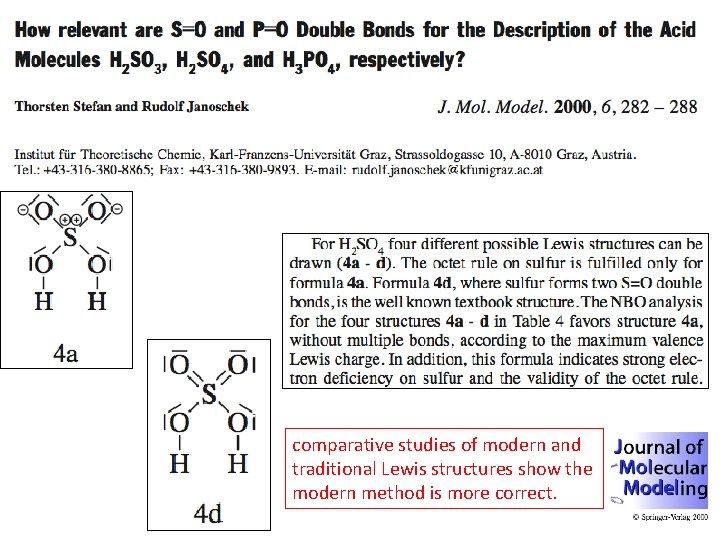
comparative studies of modern and traditional Lewis structures show the modern method is more correct.

comparative studies of modern and traditional Lewis structures show the modern method is more correct.