2 1 Ionic Compounds ionic compounds are formed







- Slides: 17

2. 1 Ionic Compounds

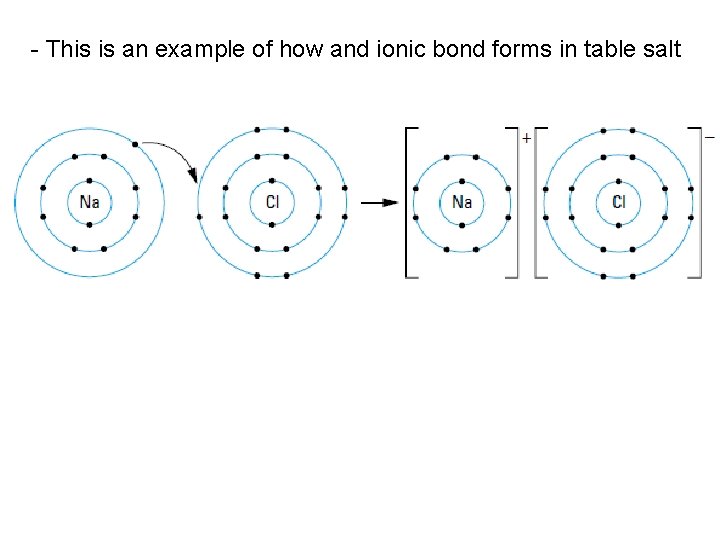
• ionic compounds are formed when one or more valence electrons are transferred from a metal atom to a nonmetal atom • leaves the metal atom as a positive ion, or cation, and the nonmetal atom as a negative ion, or anion • two oppositely charged ions are attracted to each other by a force called an ionic bond

- This is an example of how and ionic bond forms in table salt

What happens when an ionic bond forms?

Draw electron dot diagrams to illustrate the formation of magnesium oxide. Write the ion ratio and the chemical formula.

Draw electron dot diagrams to illustrate the formation of sodium phosphide. Write the ion ratio and the chemical formula.

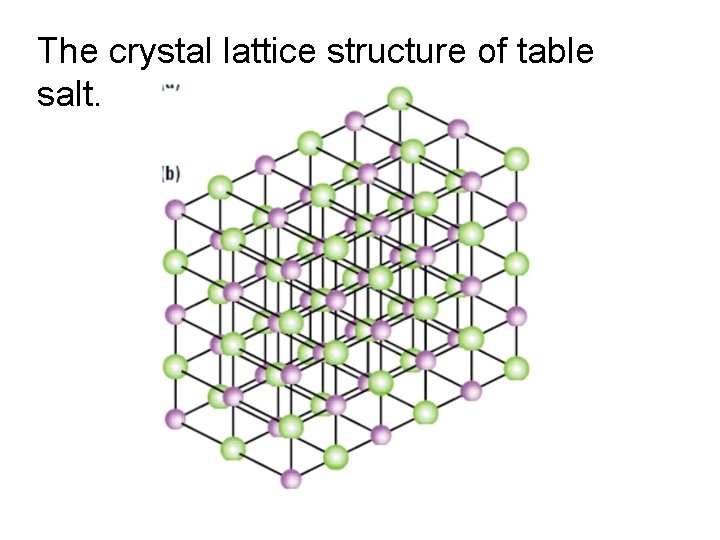
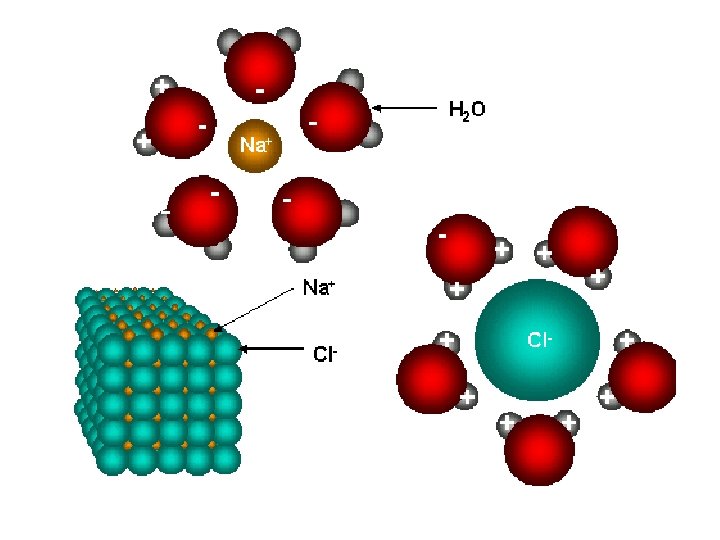
• Ionic compounds are listed in there smallest formula unit • formula unit: the simplest wholenumber ratio of atoms or ions of the elements in an ionic compound • In reality these ionic substances exist as crystal lattice structures

The crystal lattice structure of table salt.

Crystal Lattice Structures • anions and cations are locked in a regular structure by the balance of attractive bonds and electrical repulsion • Crystal lattices vary in structure • Takes a lot of energy to break the electrostatic attractions within a crystal lattice – A positive really tries to avoid being beside another positive and negative charges do the same • Once they are broken the lattice easily shatters

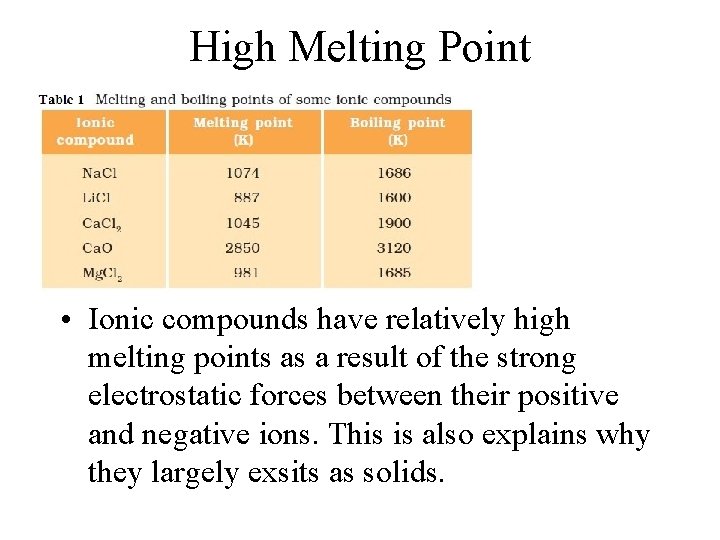
Properties of Ionic Compounds • • • solids at SATP high melting points hard brittle electrolytes

High Melting Point • Ionic compounds have relatively high melting points as a result of the strong electrostatic forces between their positive and negative ions. This is also explains why they largely exsits as solids.

Hard • They are considered hard because their bonds resist being stretched.

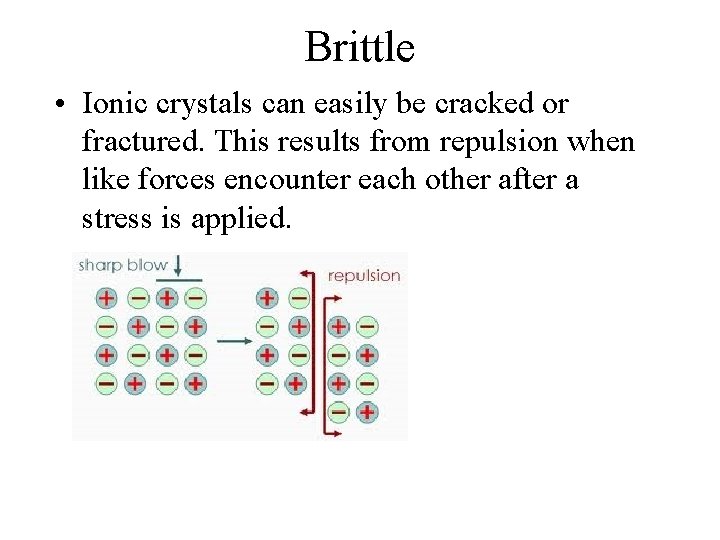
Brittle • Ionic crystals can easily be cracked or fractured. This results from repulsion when like forces encounter each other after a stress is applied.

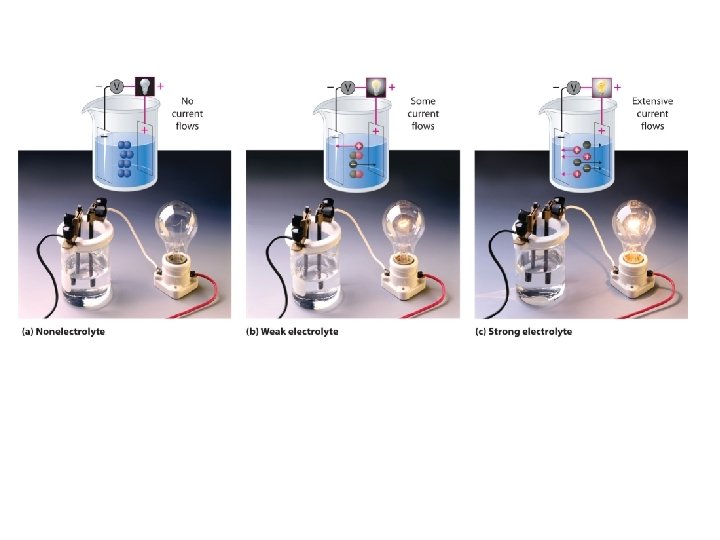
What is an Electrolyte • A subtstance that forms a solution that conducts electricity – Can be weak or strong – We can measure with a conductivity meter – They are very important in biological processes

Electrolytes • Ionic compounds are electrolytes because when they dissolve in water they separate from the crystal into their ions. The ions are then free to move and pass along electric charge. Demo-

