Polyatomic Ions Polyatomic ions a unit of more








- Slides: 11

Polyatomic Ions • Polyatomic ions- a unit of more than one atom • Ways to identify polyatomic ions…. 1. If you see elements in parentheses, these are the poly atomics Ca(OH)2 Be(NO 3)2 (NH 4)2 S 2. If you find the polyatomic ion on your polyatomic ions sheet!

Balancing Equations

Subscripts Coefficient PRODUCTS REACTANTS YIELD SYMBOL Subscripts Coefficient 2 CU + Zn(NO 3)2 2 Cu. NO 3 + Zn

Chemical Reaction Vocabulary • Chemical Reaction: One or more reactants change into one or more products • Reactant: A substance present at the start of a reaction • Product: A substance produced in a chemical reaction • Chemical Equation: An expression representing a chemical reaction; the formulas of the reactants (on the left) are connected by an arrow with the formulas for the products (on the right). • Example: Reactants Products

Law of Conservation of Mass In a chemical reaction mass is conserved. • Mass cannot be created or destroyed • It can only change forms • Solid to liquid to gas • Change into another substance

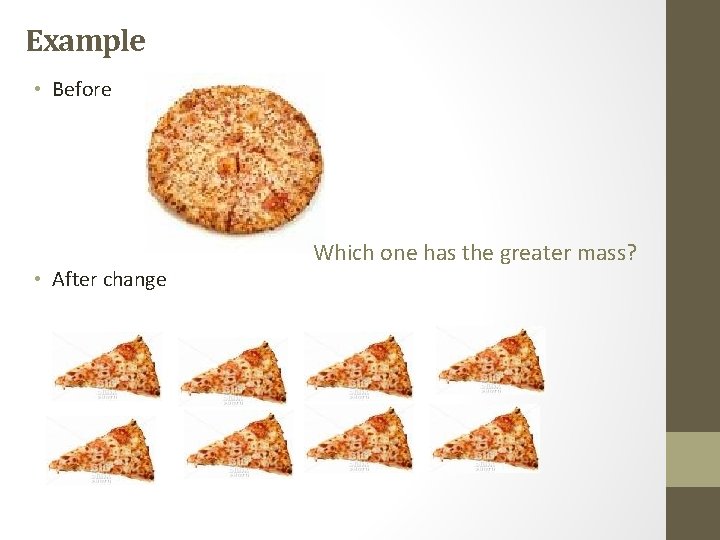
Example • Before • After change Which one has the greater mass?

Law of Conservation of Mass —Mass is never created or destroyed in a chemical reaction. • • • When material is burned, residue is less massive than original material Ash, smoke, and gases escaped into the air—(they are still considered matter. ) Their mass was not lost, only relocated due to the law of conservation of mass.

Law of Conservation of Mass We learned how to count atoms in a chemical formula, now we have to make sure we have the same amount of atoms on each side of the reaction.

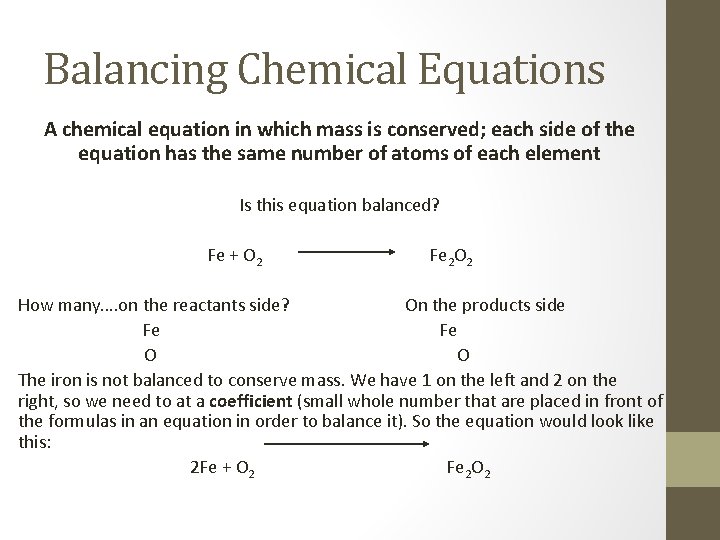
Balancing Chemical Equations A chemical equation in which mass is conserved; each side of the equation has the same number of atoms of each element Is this equation balanced? Fe + O 2 Fe 2 O 2 How many…. on the reactants side? On the products side Fe Fe O O The iron is not balanced to conserve mass. We have 1 on the left and 2 on the right, so we need to at a coefficient (small whole number that are placed in front of the formulas in an equation in order to balance it). So the equation would look like this: 2 Fe + O 2 Fe 2 O 2

Balancing Steps 1. Write the unbalanced equation. 2. Count atoms on each side. 3. Add coefficients as needed to make #s equal on both sides of the equation. Coefficient subscript = # of atoms 4. Reduce coefficients to lowest possible ratio, if necessary. 5. Double check atom balance!!!

Helpful Tips • Balance one element at a time. • Update ALL atom counts after adding a coefficient. • If an element appears more than once per side, balance it last. • Balance polyatomic ions as single units. • Common poly atomics: SO 4, PO 4, CO 3, OH • Example: “ 1 SO 4” instead of “ 1 S” and “ 4 O”