Polyatomic Ions Lesson 7 Monatomic Ions Ions that







- Slides: 13

Polyatomic Ions Lesson 7

Monatomic Ions • Ions that are composed of one atom are called monatomic ions. • Monoatomic Ion- an ion composed of only one atom. • We have only looked at these so far

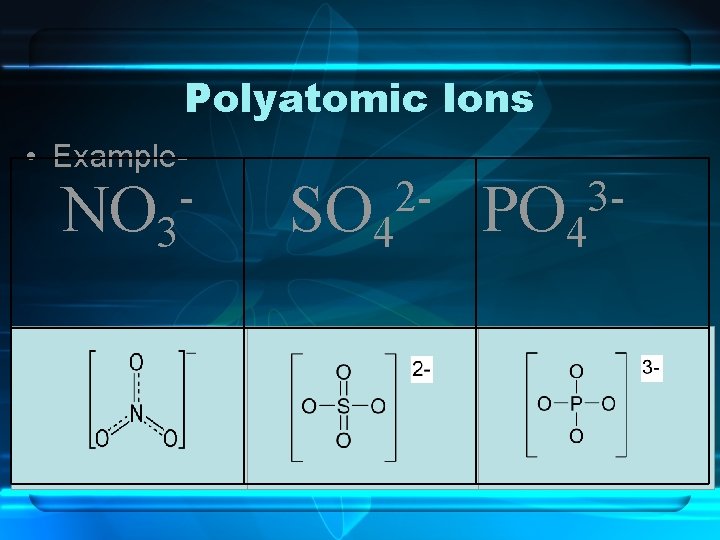
Polyatomic Ions • Polyatomic Ions are ions that are composed of more than one atom. The entire molecule carries a charge to it.

Polyatomic Ions • Example- NO 3 2 SO 4 3 PO 4

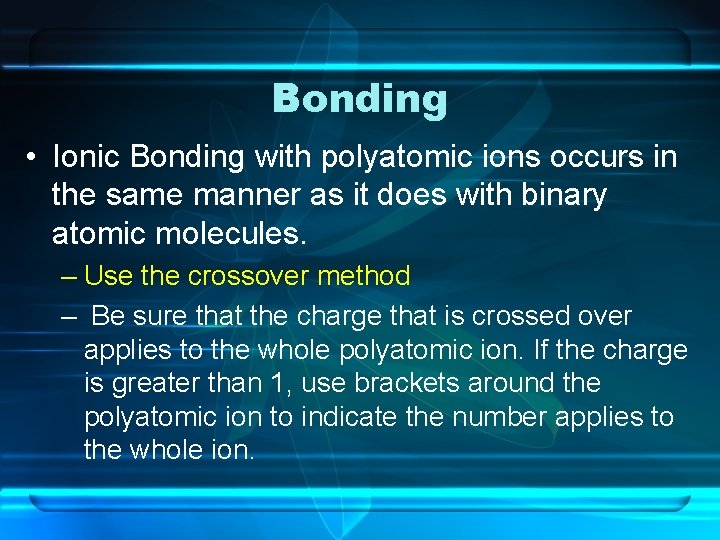
Bonding • Ionic Bonding with polyatomic ions occurs in the same manner as it does with binary atomic molecules. – Use the crossover method – Be sure that the charge that is crossed over applies to the whole polyatomic ion. If the charge is greater than 1, use brackets around the polyatomic ion to indicate the number applies to the whole ion.

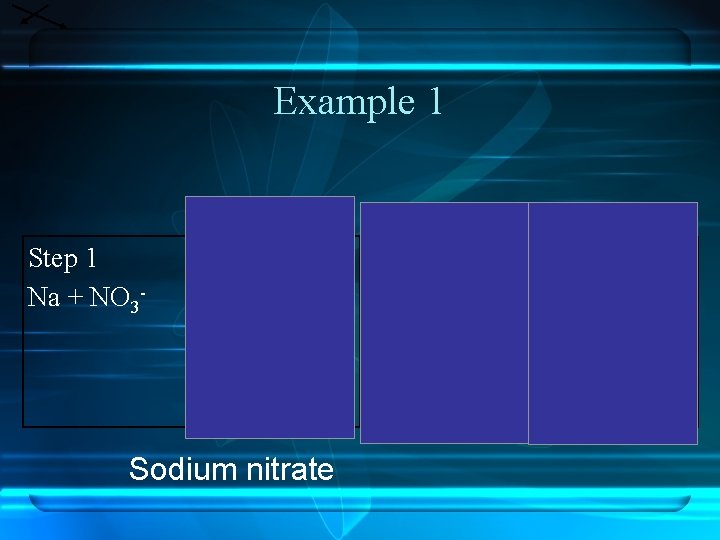
Example 1 Step 1 Na + NO 3 - Step 2 1+ Na Step 3 1 - 1+ NO 3 Na Sodium nitrate 1 NO 3 Step 4 Na. NO 3

Example 2 Step 1 Mg + NO 3 - Step 2 2+ Mg Step 3 12+ NO 3 Mg Magnesium nitrate 1 NO 3 Step 4 Mg (NO 3)2

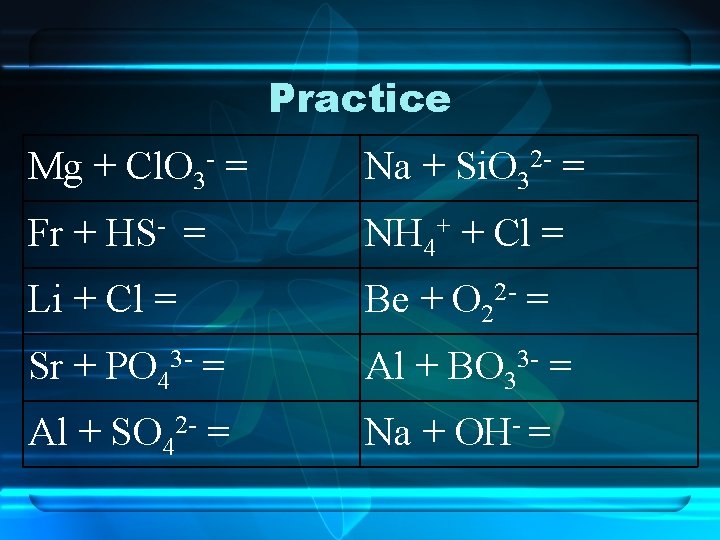
Practice Mg + Cl. O 3 - = Na + Si. O 32 - = Fr + HS- = NH 4+ + Cl = Li + Cl = Be + O 22 - = Sr + PO 43 - = Al + BO 33 - = Al + SO 42 - = Na + OH- =

Naming Ionic Polyatomic compounds: IUPAC • Multivalent: Metal (charge) polyatomic ion • Monovalent: Metal polyatomic ion • Tertiary ionic compounds are comprised of a metal ion and a polyatomic ion. Write the polyatomic ions in the same way as monatomic ions.

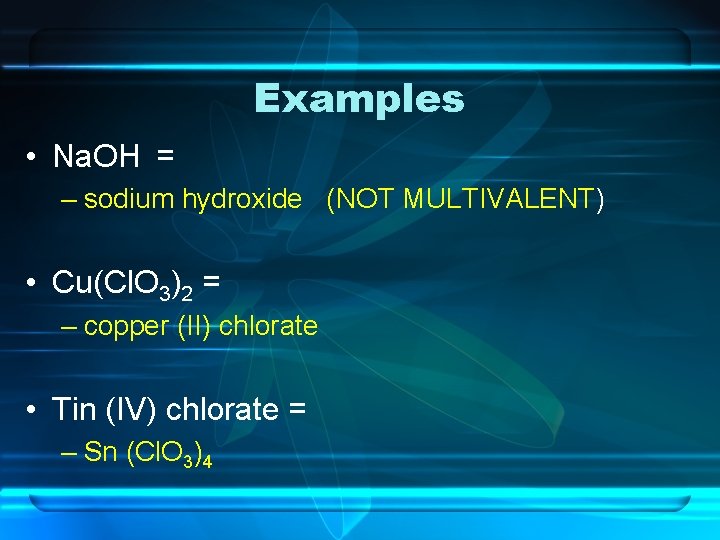
Examples • Na. OH = – sodium hydroxide (NOT MULTIVALENT) • Cu(Cl. O 3)2 = – copper (II) chlorate • Tin (IV) chlorate = – Sn (Cl. O 3)4

NOTE: • Look at the resource page, notice the * • This means that bromine and iodine will have the same ending if there are the same number of oxygen's as the chlorine.

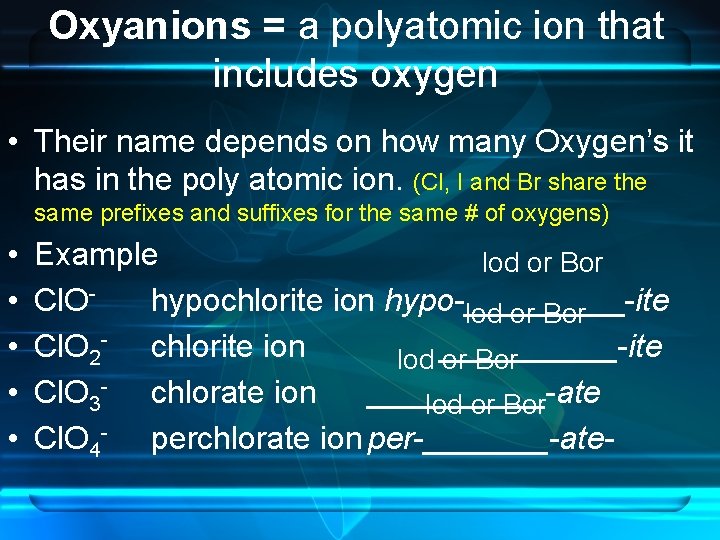
Oxyanions = a polyatomic ion that includes oxygen • Their name depends on how many Oxygen’s it has in the poly atomic ion. (Cl, I and Br share the same prefixes and suffixes for the same # of oxygens) • • • Example Iod or Bor Cl. Ohypochlorite ion hypo _____ ite Iod or Bor Cl. O 2 - chlorite ion Iod _____ ite or Bor Cl. O 3 - chlorate ion _____ ate Iod or Bor Cl. O 4 - perchlorate ion per _______ ate

Practice • Do the questions on the note.