The Nature of Covalent Bonding 8 2 Page

The Nature of Covalent Bonding 8. 2 Page 217

The Octet Rule in Covalent Bonding • In forming covalent bonds, electron sharing usually occurs so that atoms attain the electron configurations of noble gases. • A pair of hydrogen atoms share 2 electrons giving the electron configuration of helium. • Combinations of atoms of the nonmetallic elements in Groups 4 A, 5 A, 6 A, and 7 A are likely to form covalent bonds.
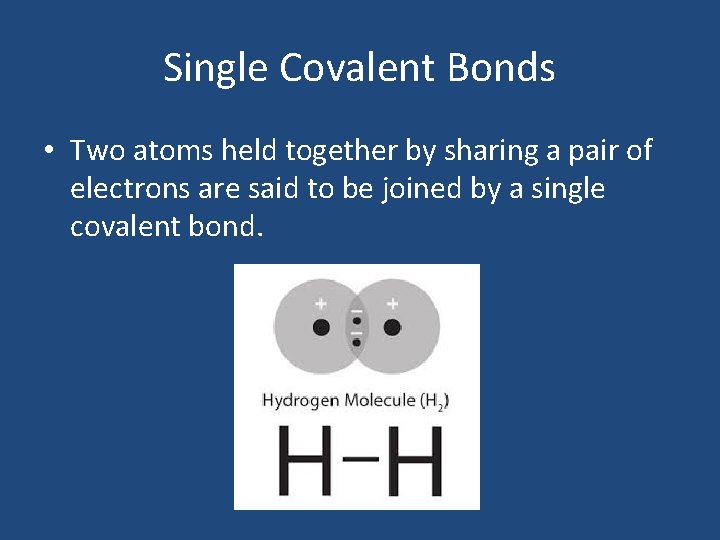
Single Covalent Bonds • Two atoms held together by sharing a pair of electrons are said to be joined by a single covalent bond.
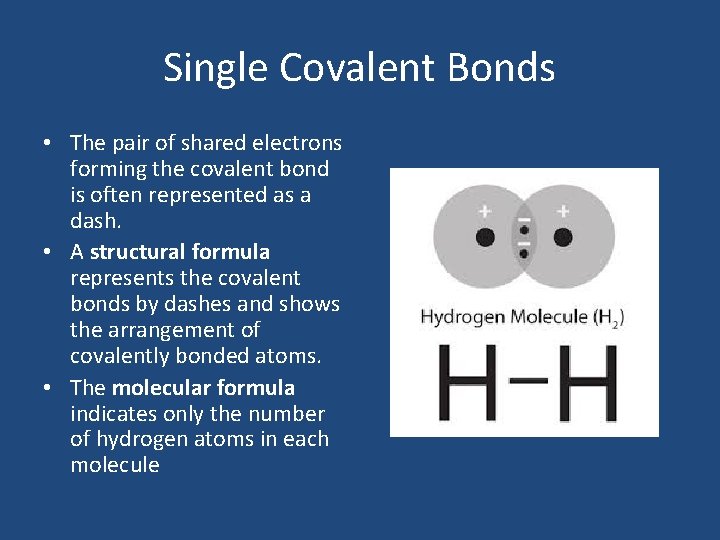
Single Covalent Bonds • The pair of shared electrons forming the covalent bond is often represented as a dash. • A structural formula represents the covalent bonds by dashes and shows the arrangement of covalently bonded atoms. • The molecular formula indicates only the number of hydrogen atoms in each molecule
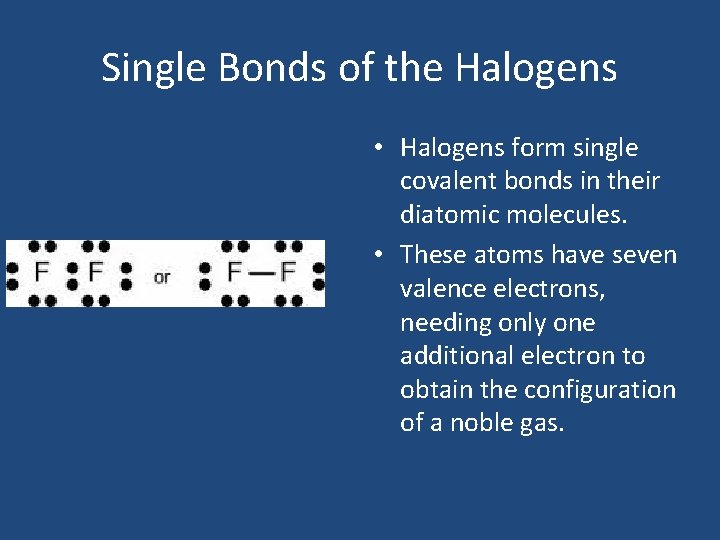
Single Bonds of the Halogens • Halogens form single covalent bonds in their diatomic molecules. • These atoms have seven valence electrons, needing only one additional electron to obtain the configuration of a noble gas.
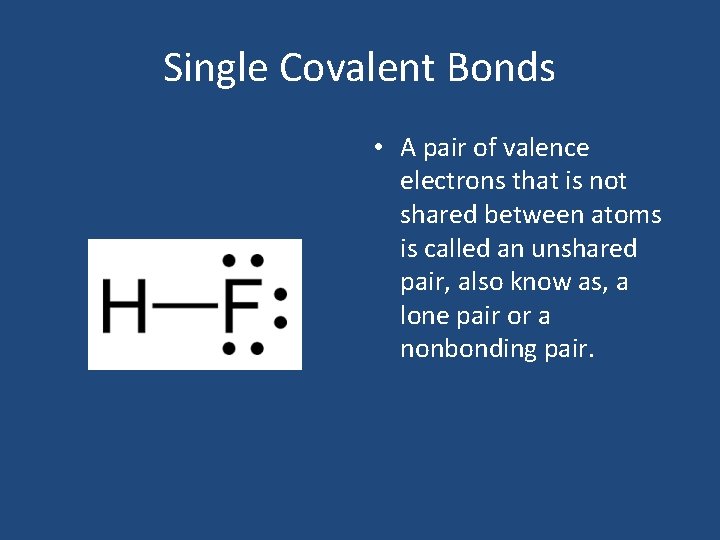
Single Covalent Bonds • A pair of valence electrons that is not shared between atoms is called an unshared pair, also know as, a lone pair or a nonbonding pair.
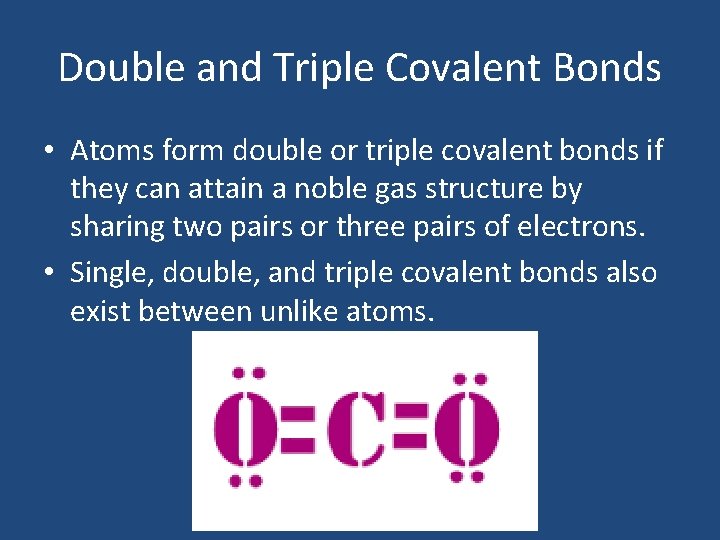
Double and Triple Covalent Bonds • Atoms form double or triple covalent bonds if they can attain a noble gas structure by sharing two pairs or three pairs of electrons. • Single, double, and triple covalent bonds also exist between unlike atoms.
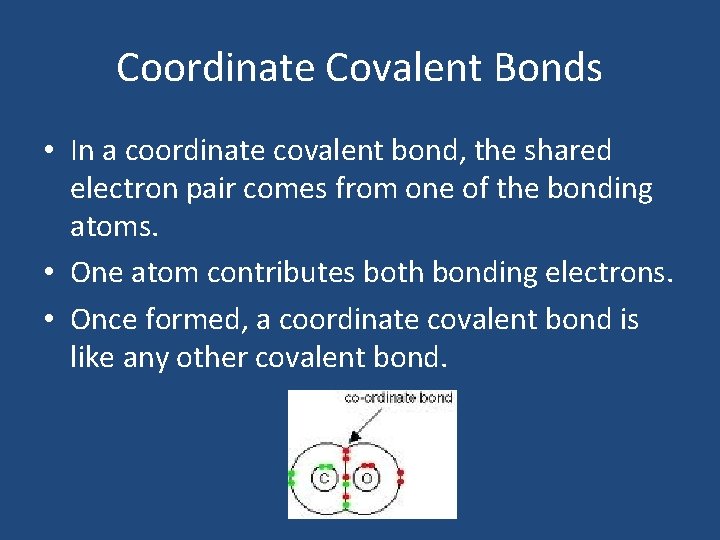
Coordinate Covalent Bonds • In a coordinate covalent bond, the shared electron pair comes from one of the bonding atoms. • One atom contributes both bonding electrons. • Once formed, a coordinate covalent bond is like any other covalent bond.

Polyatomic Ions • Tightly bound group of atoms that has a positive or negative charge and behaves as a unit. • Most polyatomic cations and anions contain both covalent and coordinate covalent bonds. • Therefore, compounds containing polyatomic ions include both ionic and covalent bonding.
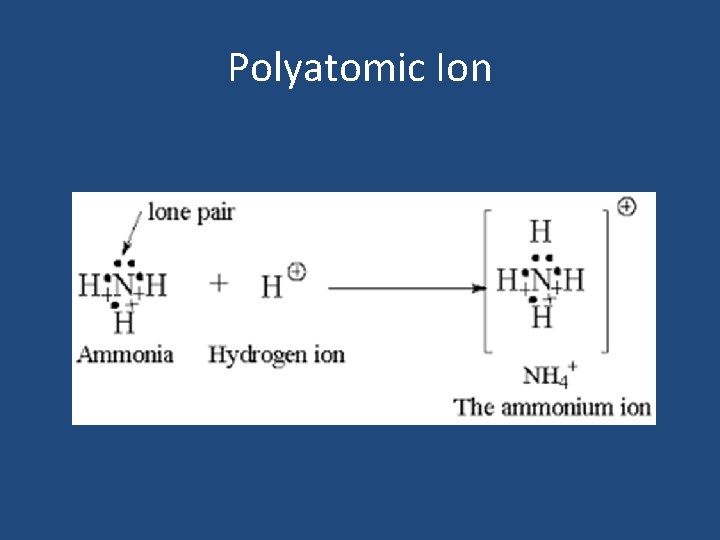
Polyatomic Ion

Bond Dissociation Energies • The energy required to break the bond between two covalently bonded atoms. • Usually expressed as the energy needed to break one mole of bonds. • A large bond dissociation energy corresponds to a strong covalent bond.

Exceptions to the Octet Rule • The octet rule cannot be satisfied in molecules whose total number of valence electrons is an odd number. There also molecules in which an atom has fewer, or more, than a complete octet of valence electrons. – Cl. O 2 – NO – Read page 228 -229
- Slides: 12