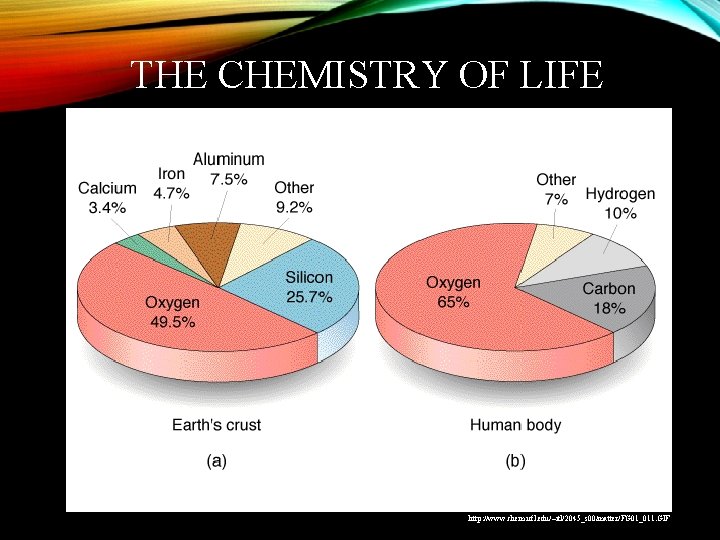
THE CHEMISTRY OF LIFE http www chem ufl

















- Slides: 73

THE CHEMISTRY OF LIFE http: //www. chem. ufl. edu/~itl/2045_s 00/matter/FG 01_011. GIF

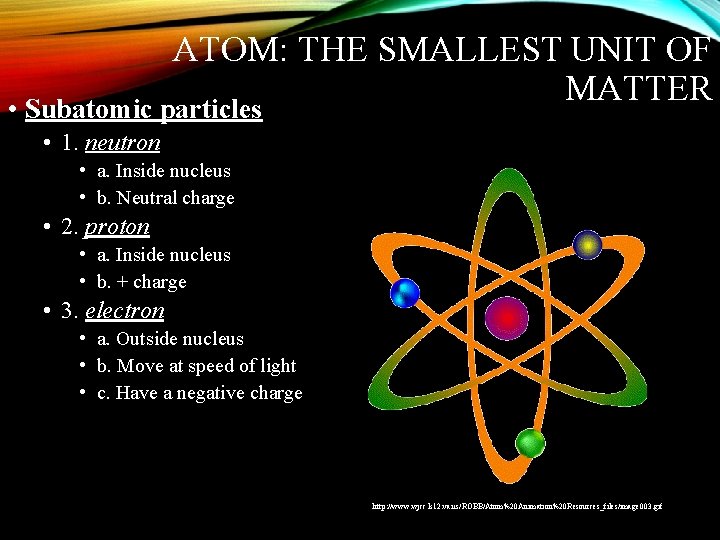
ATOM: THE SMALLEST UNIT OF MATTER • Subatomic particles • 1. neutron • a. Inside nucleus • b. Neutral charge • 2. proton • a. Inside nucleus • b. + charge • 3. electron • a. Outside nucleus • b. Move at speed of light • c. Have a negative charge http: //www. wjcc. k 12. va. us/ROBB/Atom%20 Animation%20 Resources_files/image 003. gif

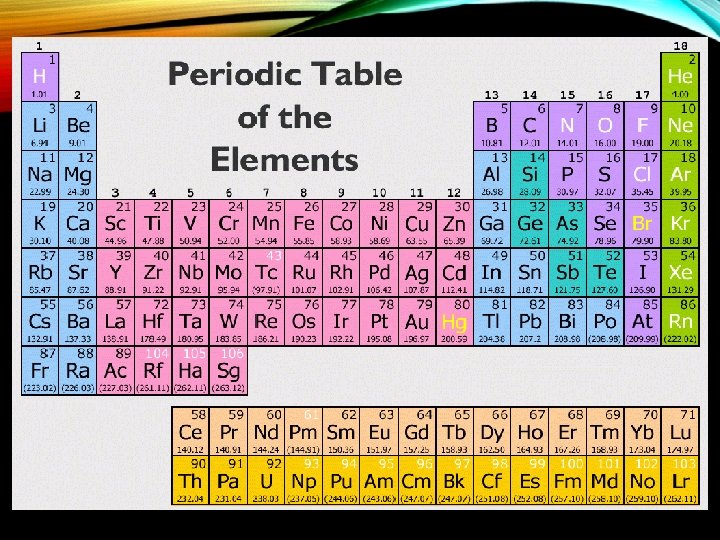
ATOMIC NUMBER AND WEIGHT • 1. atomic number- number of protons in nucleus • 2. atomic weight- number of protons plus neutrons in the nucleus of the atom (a. k. a. -mass number) http: //www. wisegorilla. com/images/chemstry/Periodic. Table. gif


HOW MANY NEUTRONS ARE IN AN ATOM? Subtract the number of protons from the mass number to get the number of neutrons Mass number – atomic number = # of neutrons

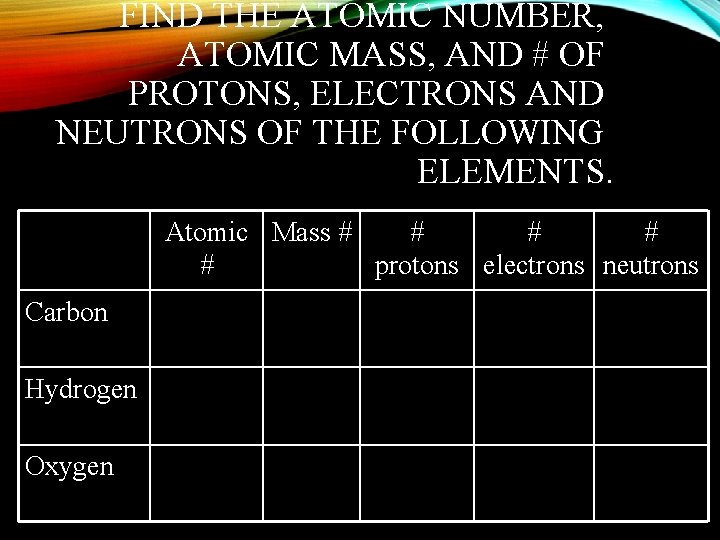
FIND THE ATOMIC NUMBER, ATOMIC MASS, AND # OF PROTONS, ELECTRONS AND NEUTRONS OF THE FOLLOWING ELEMENTS. Atomic Mass # # # protons electrons neutrons Carbon Hydrogen Oxygen

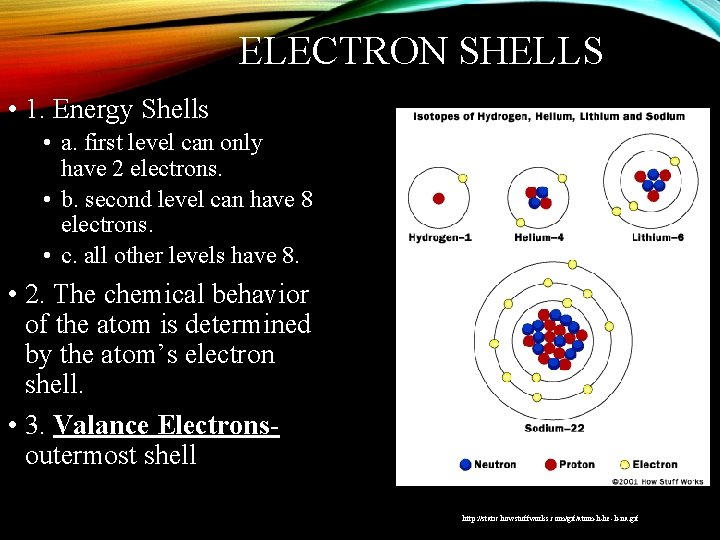
ELECTRON SHELLS • 1. Energy Shells • a. first level can only have 2 electrons. • b. second level can have 8 electrons. • c. all other levels have 8. • 2. The chemical behavior of the atom is determined by the atom’s electron shell. • 3. Valance Electronsoutermost shell http: //static. howstuffworks. com/gif/atom-h-he-li-na. gif

PROBLEM? ALL ATOMS WANT TO HAVE THEIR VALANCE ELECTRON SHELLS FULL! I wish I could be a noble gas! http: //www. csupomona. edu/~egoldstein/121/IMAGES/Periodic_noble. gif

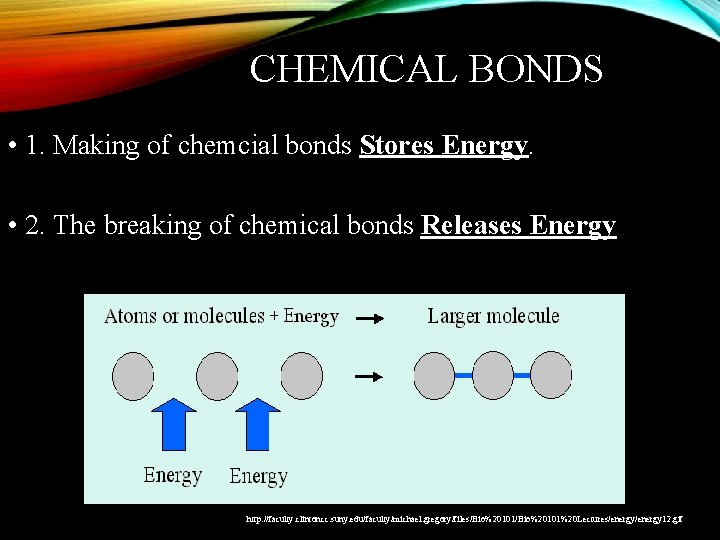
CHEMICAL BONDS • 1. Making of chemcial bonds Stores Energy. • 2. The breaking of chemical bonds Releases Energy Blah balh Sdf http: //faculty. clintoncc. suny. edu/faculty/michael. gregory/files/Bio%20101%20 Lectures/energy 12. gif

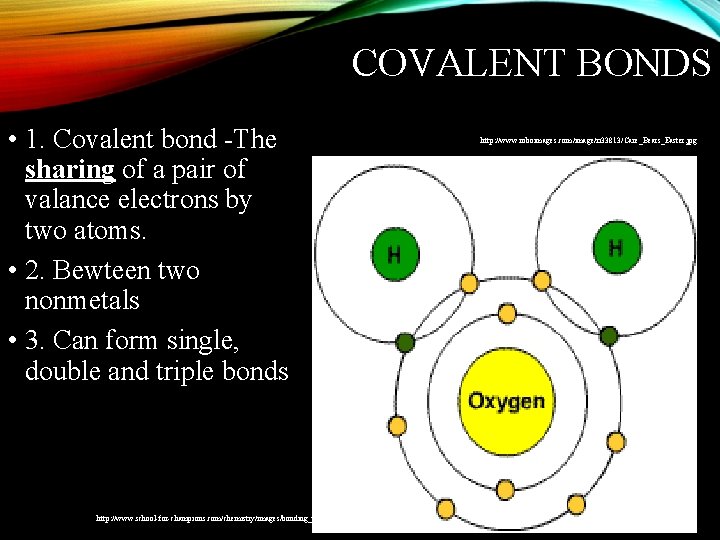
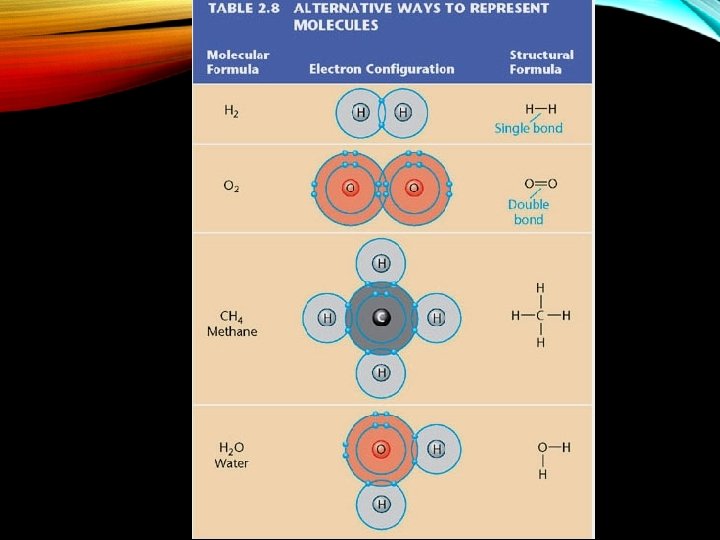
COVALENT BONDS • 1. Covalent bond -The sharing of a pair of valance electrons by two atoms. • 2. Bewteen two nonmetals • 3. Can form single, double and triple bonds http: //www. school-for-champions. com/chemistry/images/bonding_types-water. gif http: //www. roboimages. com/image/ri 33813/Care_Bears_Easter. jpg


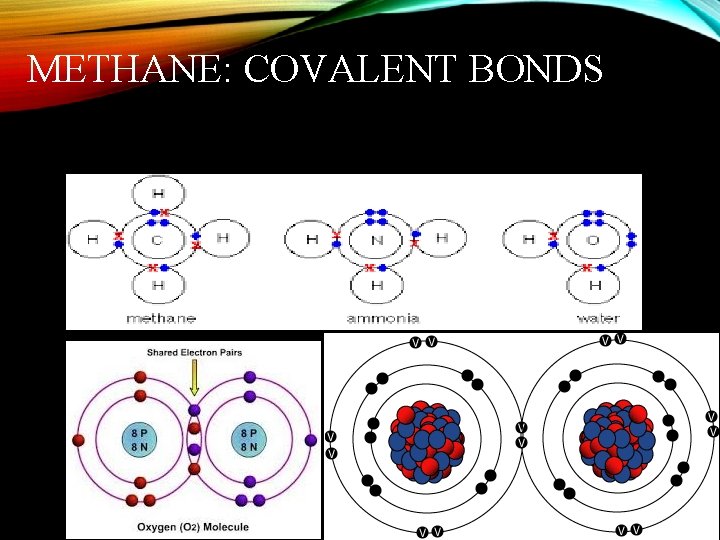
METHANE: COVALENT BONDS

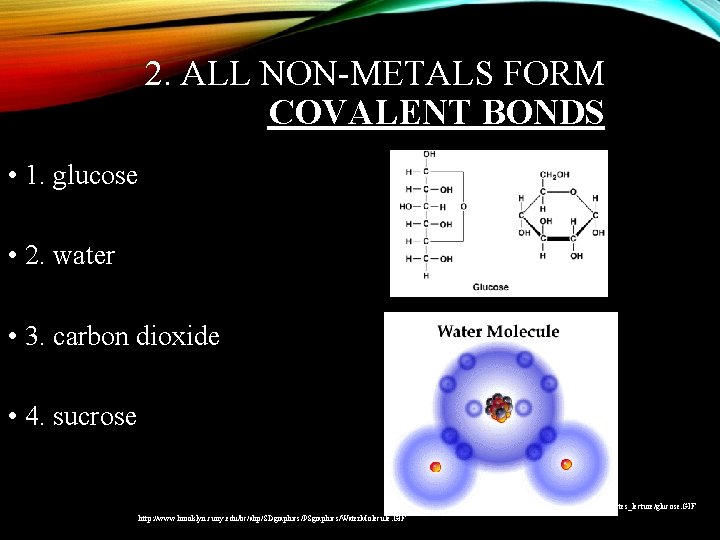
2. ALL NON-METALS FORM COVALENT BONDS • 1. glucose • 2. water • 3. carbon dioxide • 4. sucrose http: //www. peoriaendocrine. com/images/diabetes_lecture/glucose. GIF http: //www. brooklyn. cuny. edu/bc/ahp/SDgraphics/PSgraphics/Water. Molecule. GIF

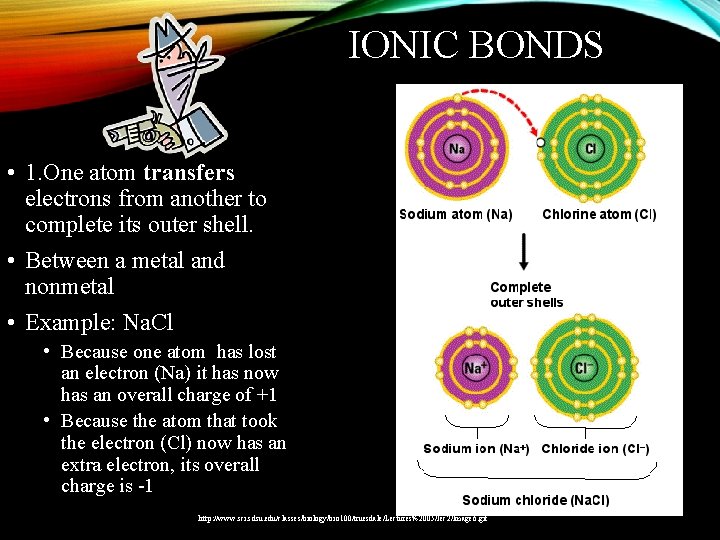
IONIC BONDS • 1. One atom transfers electrons from another to complete its outer shell. • Between a metal and nonmetal • Example: Na. Cl • Because one atom has lost an electron (Na) it has now has an overall charge of +1 • Because the atom that took the electron (Cl) now has an extra electron, its overall charge is -1 http: //www. sci. sdsu. edu/classes/biology/bio 100/truesdale/Lectures%2005/lec 2/Image 6. gif

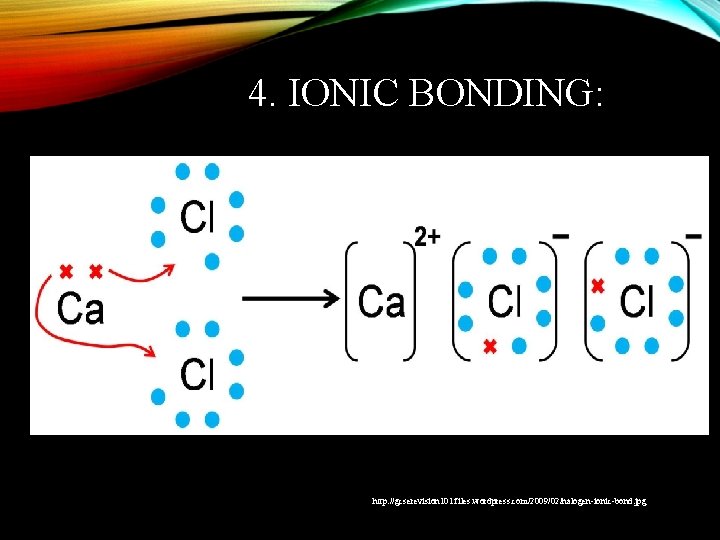
4. IONIC BONDING: http: //gcserevision 101. files. wordpress. com/2009/02/halogen-ionic-bond. jpg

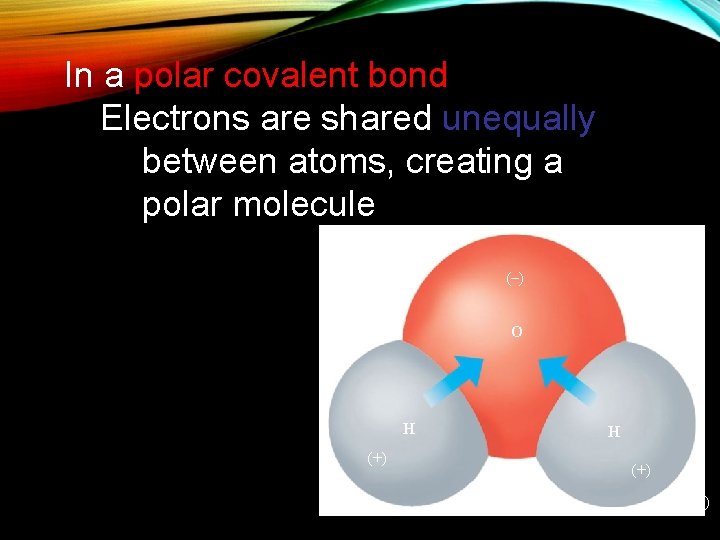
In a polar covalent bond Electrons are shared unequally between atoms, creating a polar molecule (–) (–) O H (+) (+)

STRENGTH OF BONDS • Covalent > Ionic > Hydrogen

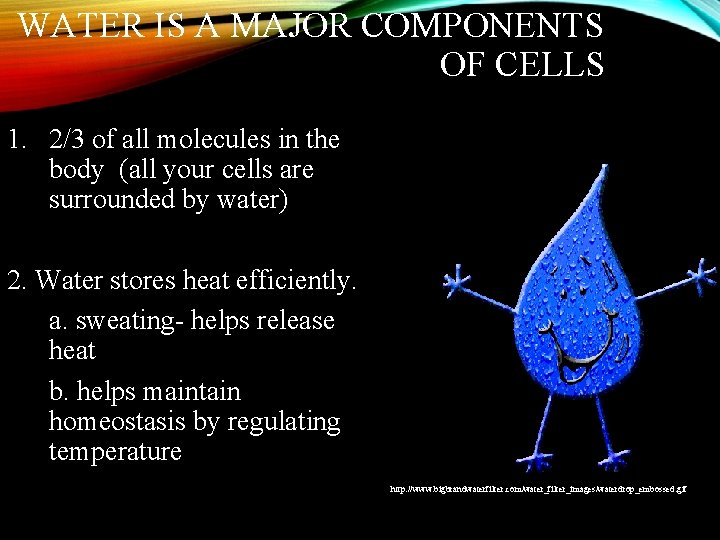
WATER IS A MAJOR COMPONENTS OF CELLS 1. 2/3 of all molecules in the body (all your cells are surrounded by water) 2. Water stores heat efficiently. a. sweating- helps release heat b. helps maintain homeostasis by regulating temperature http: //www. bigbrandwaterfilter. com/water_filter_images/waterdrop_embossed. gif

Properties of Water 1. Water is the solvent of life (universal solvent) - Polar solutes dissolve when water molecules surround them, forming aqueous solutions 2. Water is less dense as a solid than a liquid. Ice floats on water. (Ex. Glacier)

. Hydrogen bonds make water cohesive (water sticks to water) ex: Insects can walk on water due to surface tension (cohesion of water molecules at the surface of a body of water)

Water is adhesive (water sticks to other substances) ex: molecules can move from a plant’s roots to its leaves (Capillary Action); water moves up a straw

WATER DISSOLVES MANY SUBSTANCES • Water is the solvent. What it is dissolving is called the solute. • Solution- mixture in which one or more substances is evenly distributed. Solute and Solvent together make a Solution. http: //www. elmhurst. edu/~chm/demos/images/bluebottle. GIF

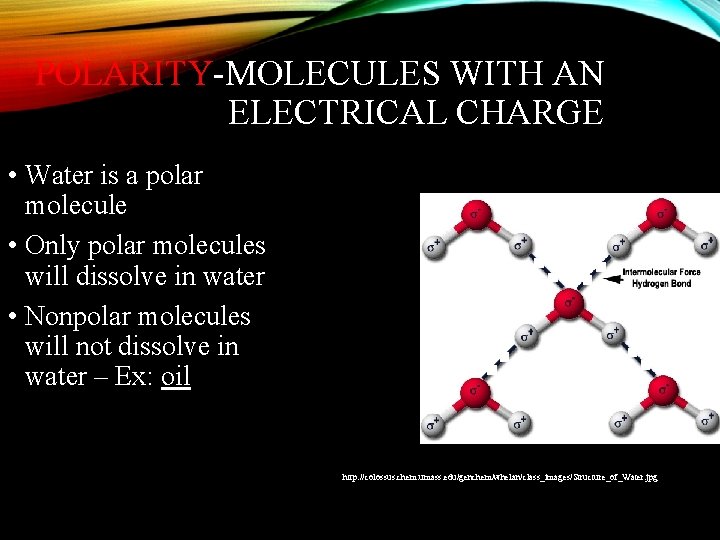
POLARITY-MOLECULES WITH AN ELECTRICAL CHARGE • Water is a polar molecule • Only polar molecules will dissolve in water • Nonpolar molecules will not dissolve in water – Ex: oil http: //colossus. chem. umass. edu/genchem/whelan/class_images/Structure_of_Water. jpg

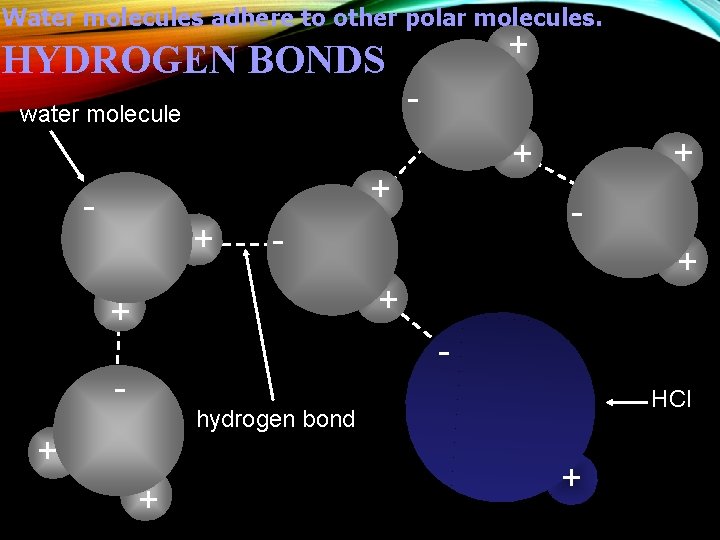
Water molecules adhere to other polar molecules. HYDROGEN BONDS water molecule + - + + + - - HCl hydrogen bond + + +

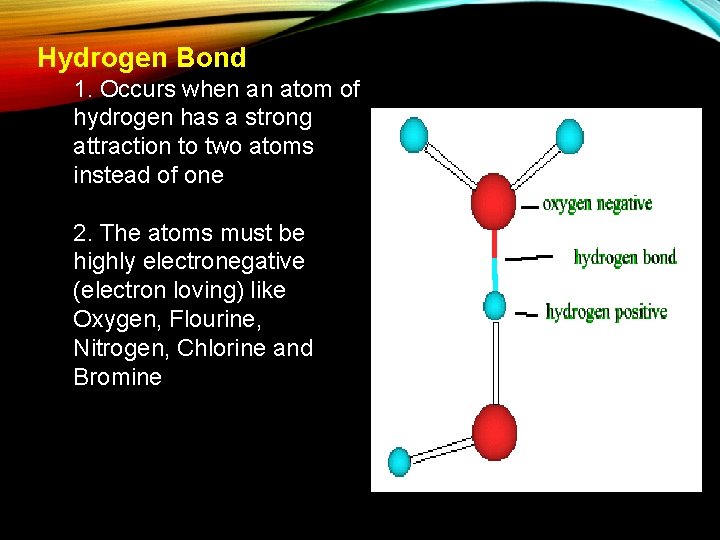
Hydrogen Bond 1. Occurs when an atom of hydrogen has a strong attraction to two atoms instead of one 2. The atoms must be highly electronegative (electron loving) like Oxygen, Flourine, Nitrogen, Chlorine and Bromine

Water has high specific heat. Specific Heat is the amount of energy required to change the temperature of a substance. It allows for moderation of climate and helps organisms regulate body temperature.

Water has a high heat of vaporization (the amount of heat required to convert liquid water into gaseous water, aka steam). This makes it an effective coolant for the body. That is why sweating actually cools us down. (Evaporative Coolant)

Water has a high boiling point (100 degrees Celsius) and low freezing point (0 degrees Celsius) Water has a neutral p. H. Which makes is a good buffer. A buffer is a substance that helps to moderate any changes in p. H that result from the addition of acids or bases.

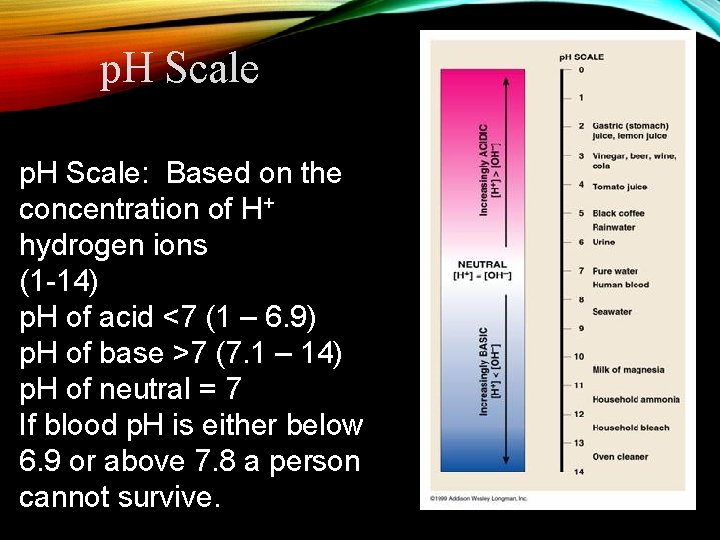
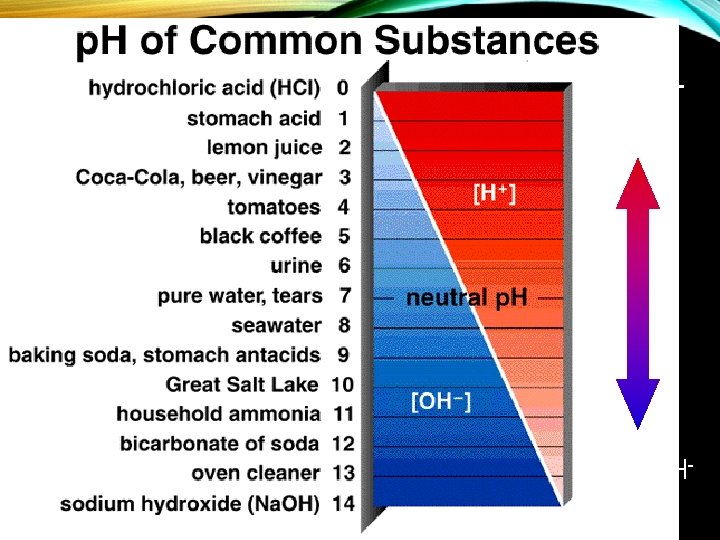
p. H Scale: Based on the concentration of H+ hydrogen ions (1 -14) p. H of acid <7 (1 – 6. 9) p. H of base >7 (7. 1 – 14) p. H of neutral = 7 If blood p. H is either below 6. 9 or above 7. 8 a person cannot survive.

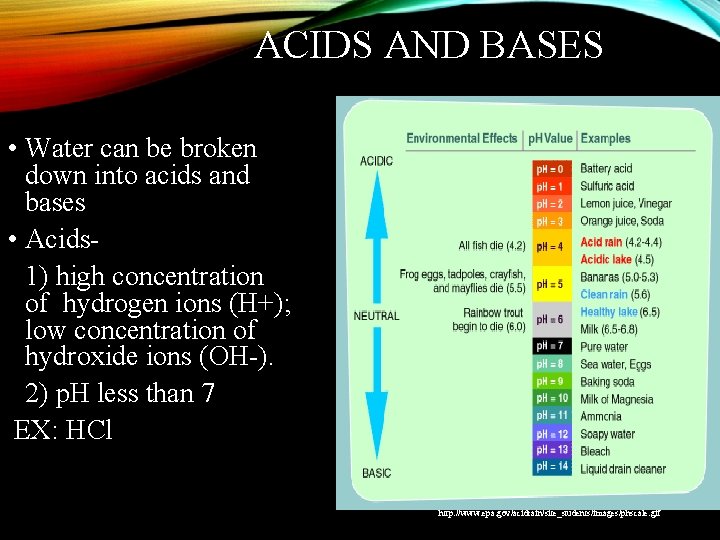
ACIDS AND BASES • Water can be broken down into acids and bases • Acids 1) high concentration of hydrogen ions (H+); low concentration of hydroxide ions (OH-). 2) p. H less than 7 EX: HCl http: //www. epa. gov/acidrain/site_students/images/phscale. gif

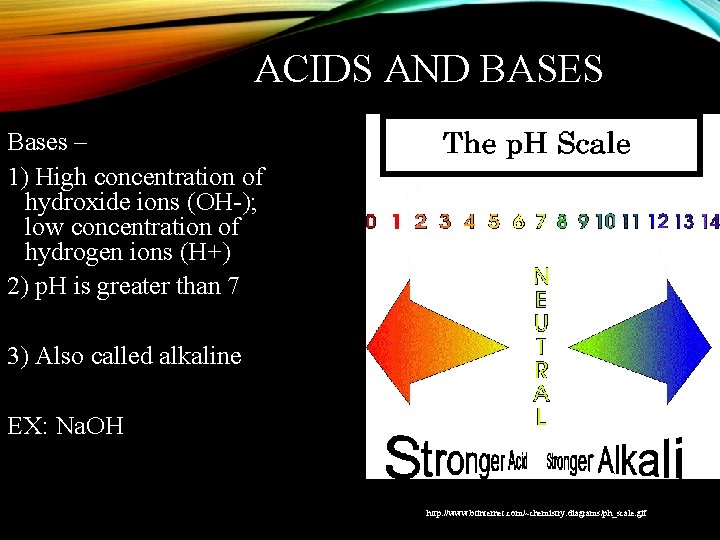
ACIDS AND BASES Bases – 1) High concentration of hydroxide ions (OH-); low concentration of hydrogen ions (H+) 2) p. H is greater than 7 3) Also called alkaline EX: Na. OH http: //www. btinternet. com/~chemistry. diagrams/ph_scale. gif

PH (2) More H+ More OH-

ORGANIC COMPOUNDS • Contain carbon usually bonded to oxygen, hydrogen, and other carbon atoms. • Most of the matter in your body is organic! • These are compounds that usually come from organisms http: //www. chemistryland. com/Elementary. School/Building. Blocks/Jungle 500. jpg

Macromolecules Cells and their organelles are made up of smaller building blocks called macromolecules. There are 4 basic types of macromolecules. They are: Lipids Proteins Carbohydrates Nucleic Acids

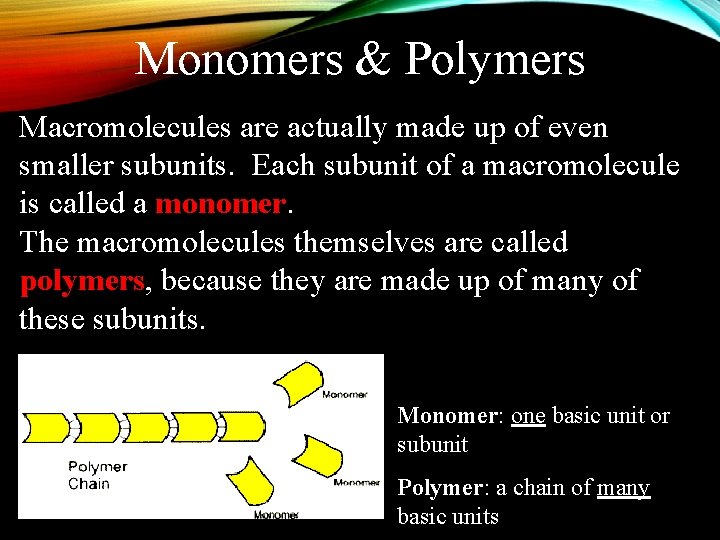
Monomers & Polymers Macromolecules are actually made up of even smaller subunits. Each subunit of a macromolecule is called a monomer. The macromolecules themselves are called polymers, because they are made up of many of these subunits. Monomer: one basic unit or subunit Polymer: a chain of many basic units

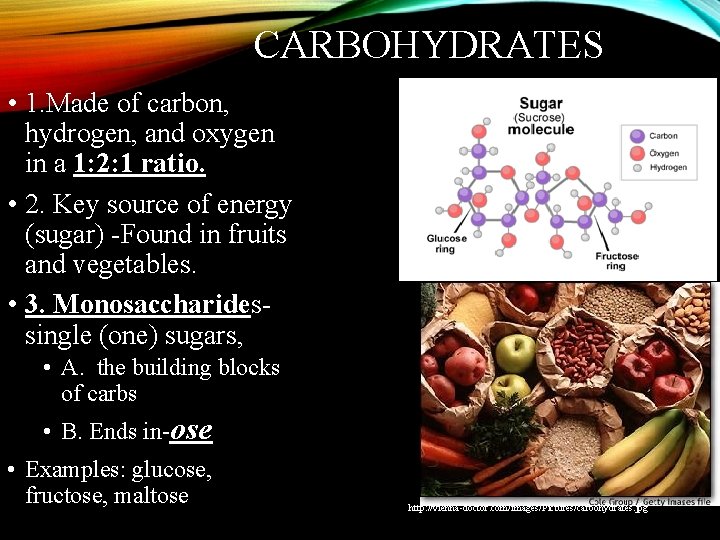
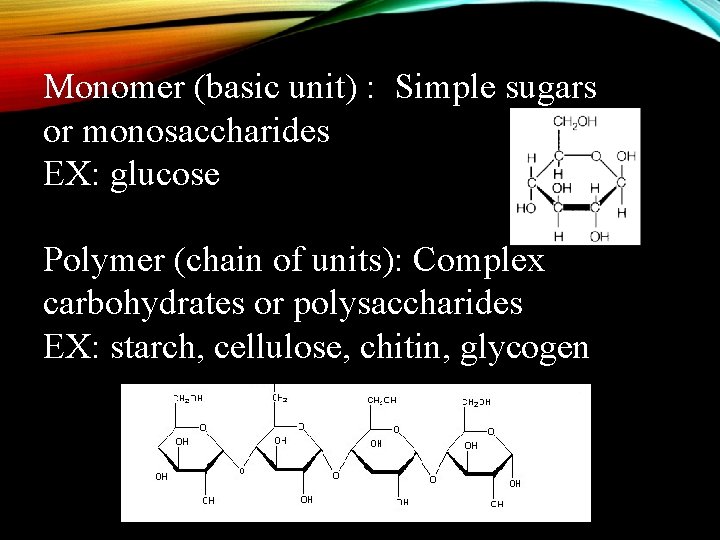
CARBOHYDRATES • 1. Made of carbon, hydrogen, and oxygen in a 1: 2: 1 ratio. • 2. Key source of energy (sugar) -Found in fruits and vegetables. • 3. Monosaccharidessingle (one) sugars, • A. the building blocks of carbs • B. Ends in-ose • Examples: glucose, fructose, maltose http: //vienna-doctor. com/images/Pictures/carbohydrates. jpg

Monomer (basic unit) : Simple sugars or monosaccharides EX: glucose Polymer (chain of units): Complex carbohydrates or polysaccharides EX: starch, cellulose, chitin, glycogen

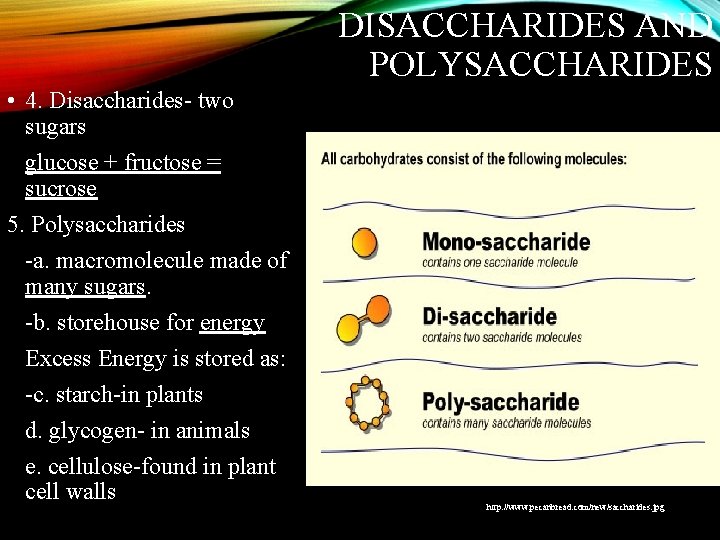
DISACCHARIDES AND POLYSACCHARIDES • 4. Disaccharides- two sugars glucose + fructose = sucrose 5. Polysaccharides -a. macromolecule made of many sugars. -b. storehouse for energy Excess Energy is stored as: -c. starch-in plants d. glycogen- in animals e. cellulose-found in plant cell walls http: //www. pecanbread. com/new/saccharides. jpg

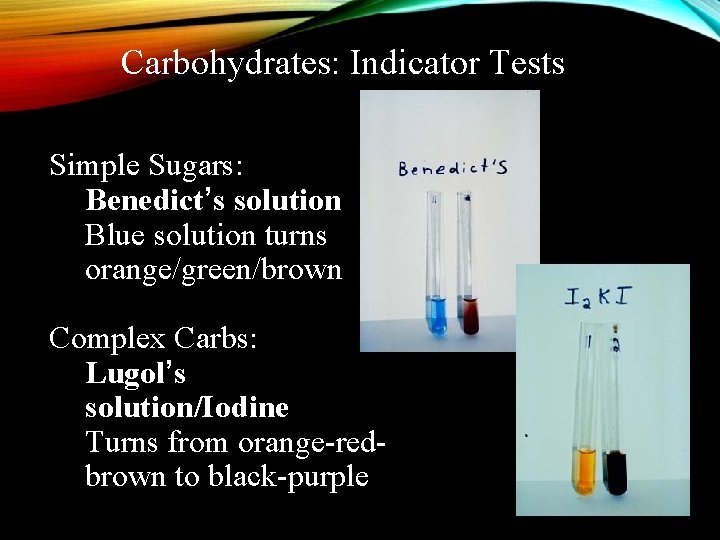
Carbohydrates: Indicator Tests Simple Sugars: Benedict’s solution Blue solution turns orange/green/brown Complex Carbs: Lugol’s solution/Iodine Turns from orange-redbrown to black-purple

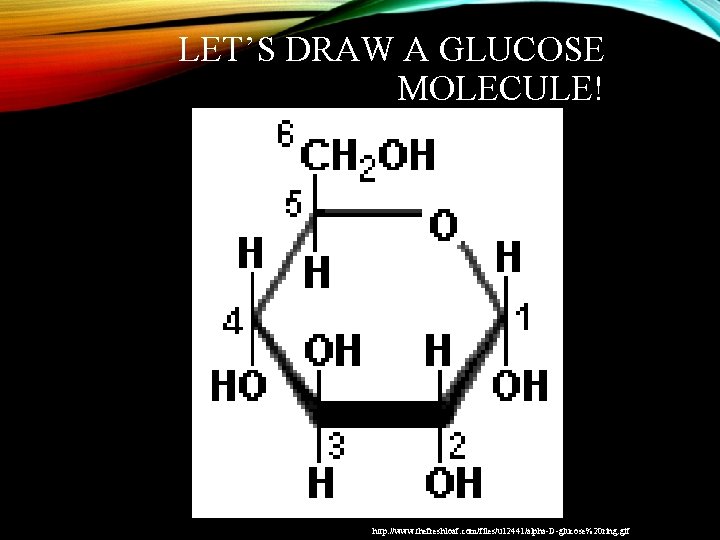
LET’S DRAW A GLUCOSE MOLECULE! http: //www. thefreshloaf. com/files/u 12441/alpha-D-glucose%20 ring. gif

LIPIDS- ARE NONPOLAR • Fats, phospholipids, oils, steroids(cholesterol) and waxes. • Fats are lipids that store energy for long term, make up the cell membrane (phospholipds), provide cell structure, provide insulation http: //www. bodybuilding. com/fun/crisco 1 k. jpg http: //cwx. prenhall. com/bookbind/pubbooks/hillchem 3/medialib/media_portfolio/text_images/CH 09/FG 09_16 -05 Box. JPG http: //www. healingtouchwebhelp. net/image/heart 31. jpg http: //www. chemistryland. com/Elementary. School/Building. Blocks/Lipids. jpg

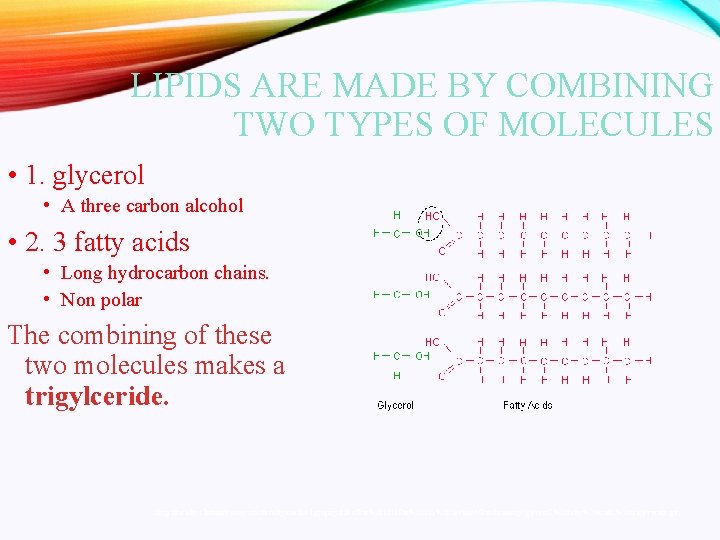
LIPIDS ARE MADE BY COMBINING TWO TYPES OF MOLECULES • 1. glycerol • A three carbon alcohol • 2. 3 fatty acids • Long hydrocarbon chains. • Non polar The combining of these two molecules makes a trigylceride. http: //faculty. clintoncc. suny. edu/faculty/michael. gregory/files/Bio%20101%20 Lectures/Biochemistry/glycerol, %20 fatty%20 acids, %20 triglyceride. gif

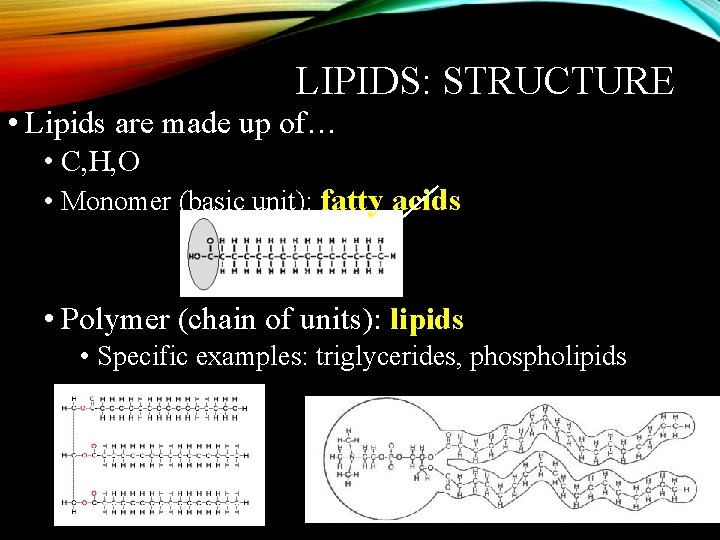
LIPIDS: STRUCTURE • Lipids are made up of… • C, H, O • Monomer (basic unit): fatty acids • Polymer (chain of units): lipids • Specific examples: triglycerides, phospholipids

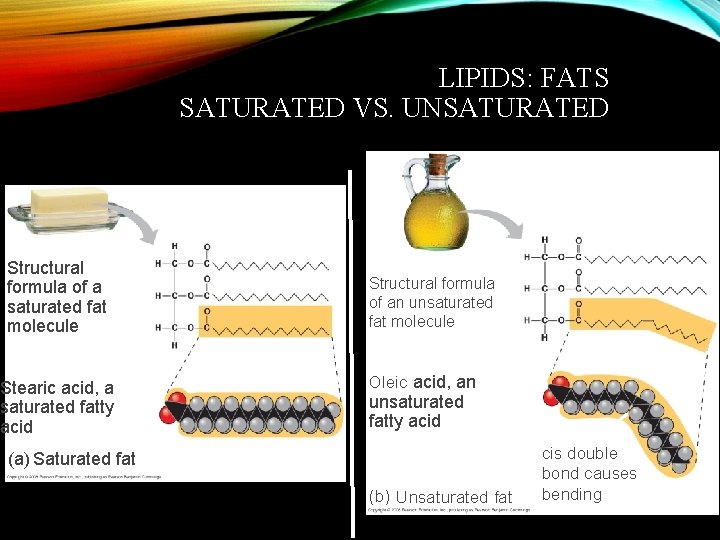
LIPIDS: FATS SATURATED VS. UNSATURATED Structural formula of a saturated fat molecule Stearic acid, a saturated fatty acid Structural formula of an unsaturated fat molecule Oleic acid, an unsaturated fatty acid (a) Saturated fat (b) Unsaturated fat cis double bond causes bending

LIPIDS: INDICATOR TEST • Paper Bag Test: • Smear substance onto paper bag • If see-thru, it contains lipids

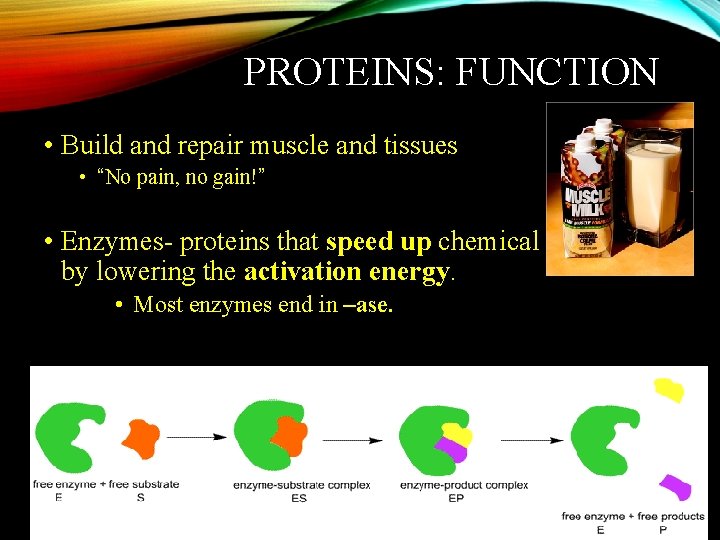
PROTEINS: FUNCTION • Build and repair muscle and tissues • “No pain, no gain!” • Enzymes- proteins that speed up chemical reactions by lowering the activation energy. • Most enzymes end in –ase.

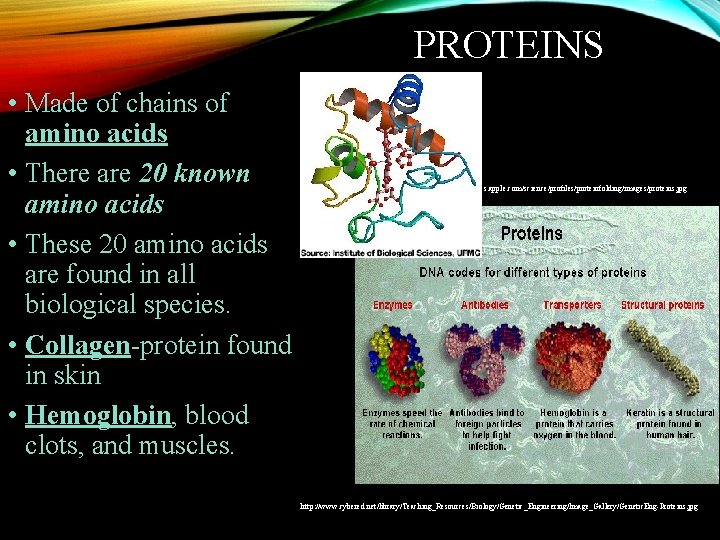
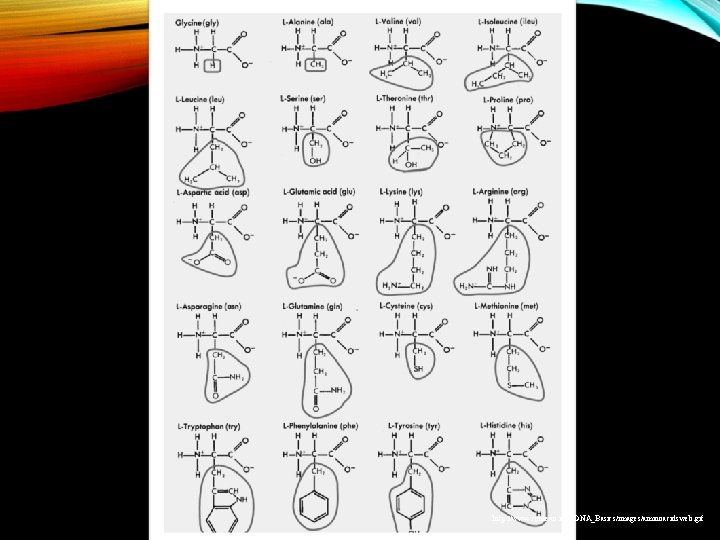
PROTEINS • Made of chains of amino acids • There are 20 known amino acids • These 20 amino acids are found in all biological species. • Collagen-protein found in skin • Hemoglobin, blood clots, and muscles. http: //images. apple. com/science/profiles/proteinfolding/images/proteins. jpg http: //www. cybered. net/library/Teaching_Resources/Biology/Genetic_Engineering/Image_Gallery/Genetic. Eng-Proteins. jpg

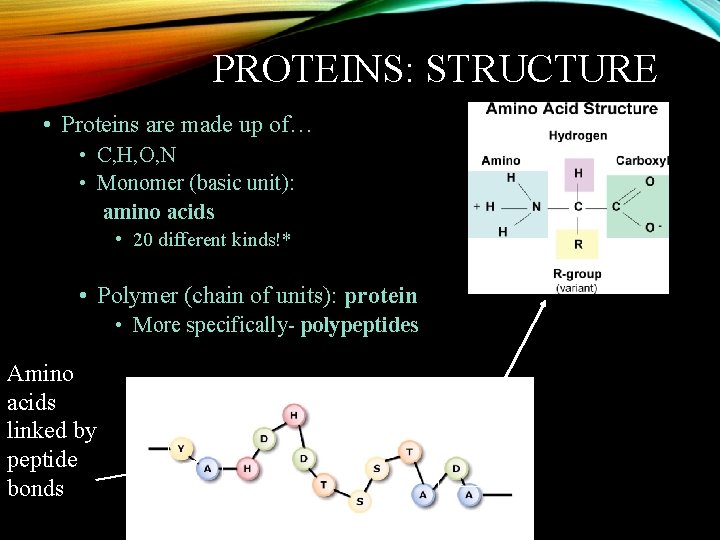
PROTEINS: STRUCTURE • Proteins are made up of… • C, H, O, N • Monomer (basic unit): amino acids • 20 different kinds!* • Polymer (chain of units): protein • More specifically- polypeptides Amino acids linked by peptide bonds dipeptide

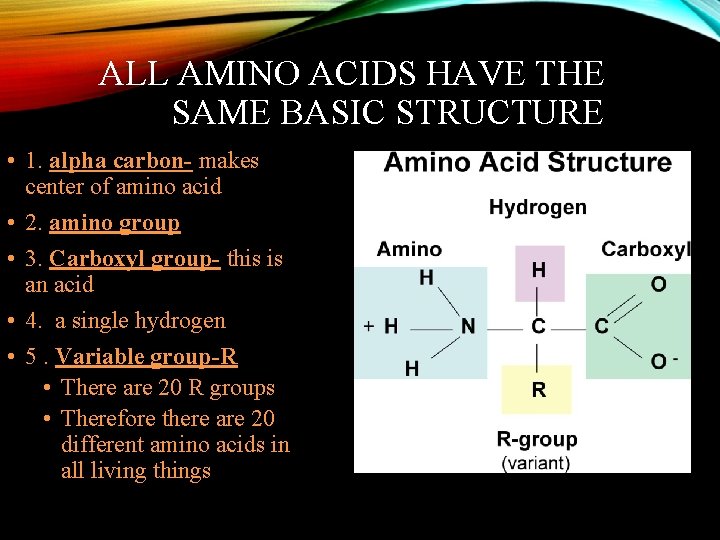
ALL AMINO ACIDS HAVE THE SAME BASIC STRUCTURE • 1. alpha carbon- makes center of amino acid • 2. amino group • 3. Carboxyl group- this is an acid • 4. a single hydrogen • 5. Variable group-R • There are 20 R groups • Therefore there are 20 different amino acids in all living things

http: //www. contexo. info/DNA_Basics/images/aminoacidsweb. gif

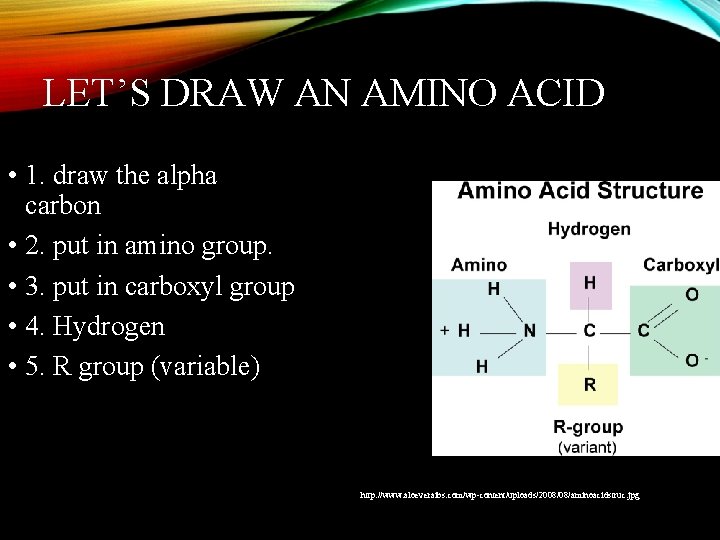
LET’S DRAW AN AMINO ACID • 1. draw the alpha carbon • 2. put in amino group. • 3. put in carboxyl group • 4. Hydrogen • 5. R group (variable) http: //www. aloeveraibs. com/wp-content/uploads/2008/08/aminoacidstruc. jpg

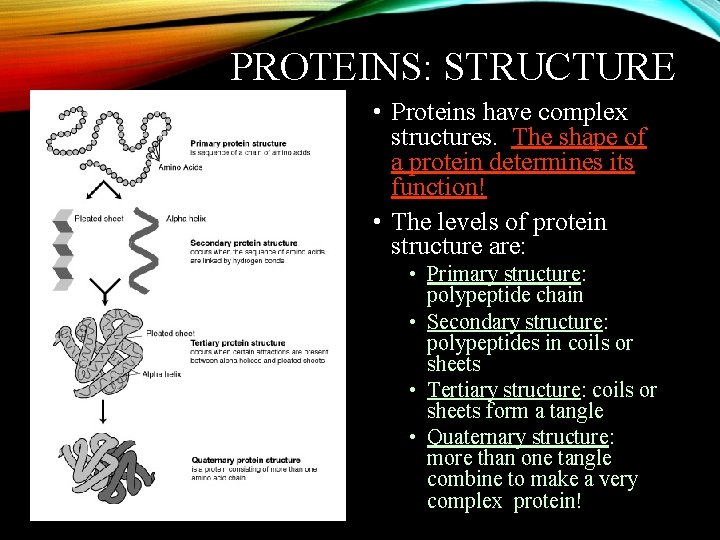
PROTEINS: STRUCTURE • Proteins have complex structures. The shape of a protein determines its function! • The levels of protein structure are: • Primary structure: polypeptide chain • Secondary structure: polypeptides in coils or sheets • Tertiary structure: coils or sheets form a tangle • Quaternary structure: more than one tangle combine to make a very complex protein!

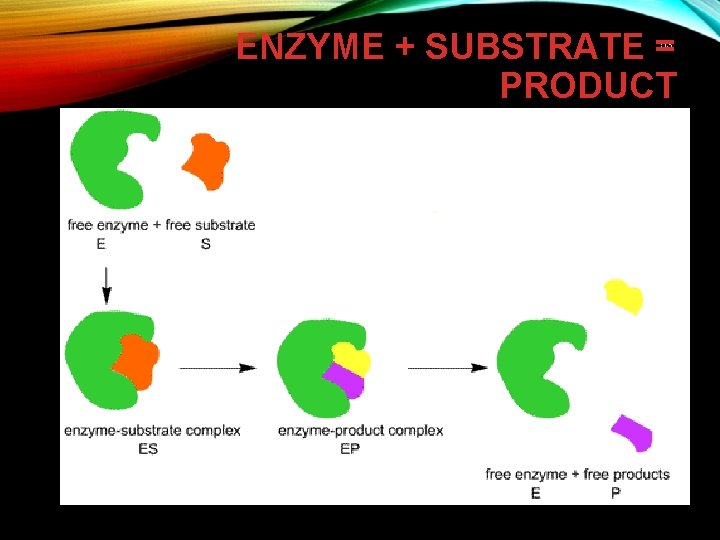
ENZYME + SUBSTRATE = PRODUCT 53

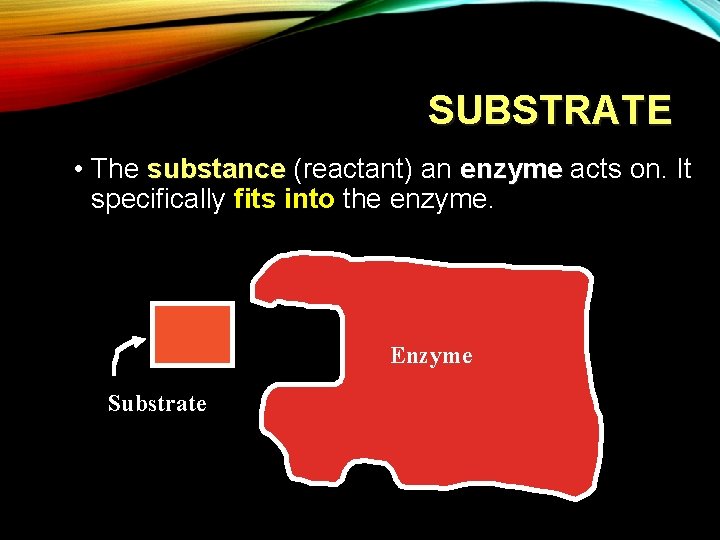
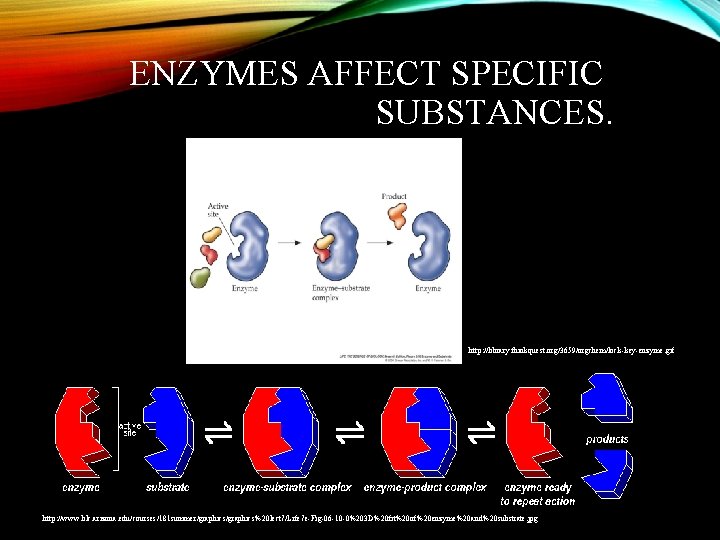
SUBSTRATE • The substance (reactant) an enzyme acts on. It specifically fits into the enzyme. Enzyme Substrate

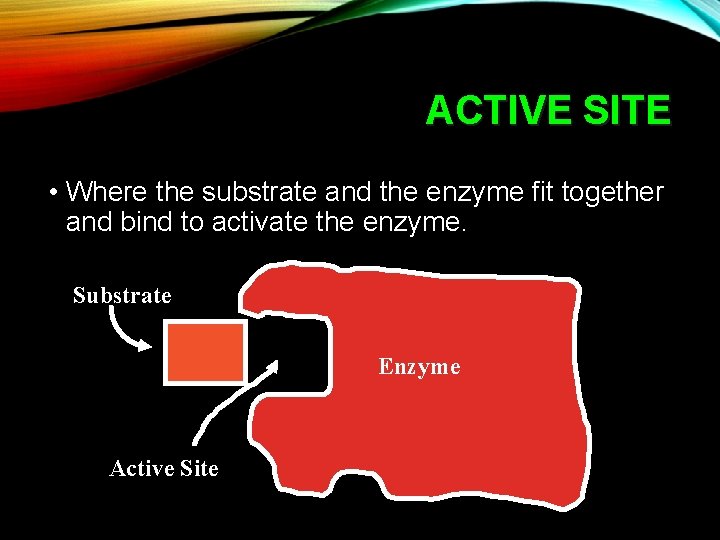
ACTIVE SITE • Where the substrate and the enzyme fit together and bind to activate the enzyme. Substrate Enzyme Active Site

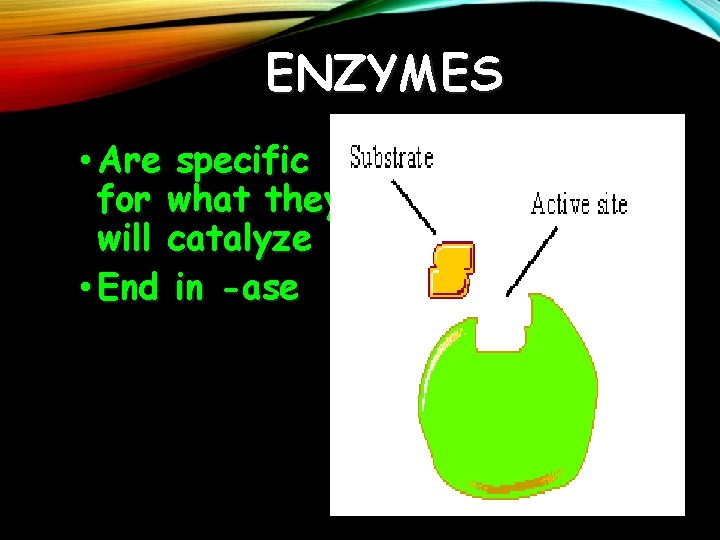
ENZYMES • Are specific for what they will catalyze • End in -ase

ENZYMES ARE SPECIFIC, LIKE A LOCK & KEY

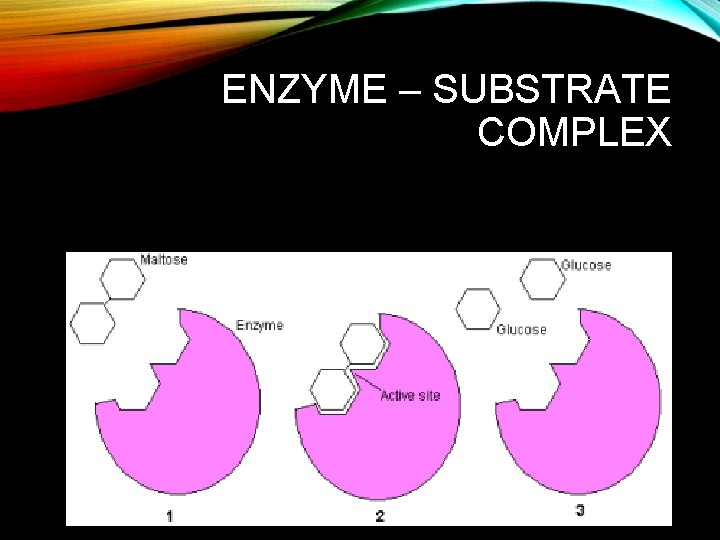
ENZYME – SUBSTRATE COMPLEX

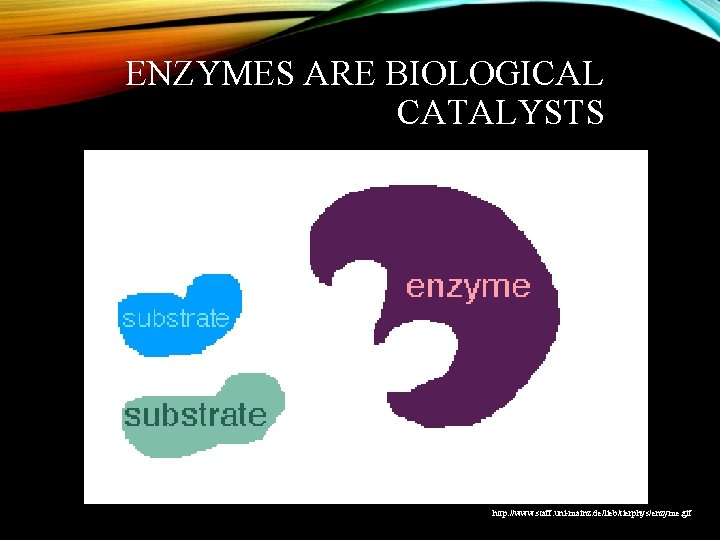
ENZYMES ARE BIOLOGICAL CATALYSTS http: //www. staff. uni-mainz. de/lieb/tierphys/enzyme. gif

ENZYMES AFFECT SPECIFIC SUBSTANCES. http: //library. thinkquest. org/3659/orgchem/lock-key-enzyme. gif http: //www. blc. arizona. edu/courses/181 summer/graphics%20 lect 7/Life 7 e-Fig-06 -10 -0%203 D%20 fit%20 of%20 enzyme%20 and%20 substrate. jpg

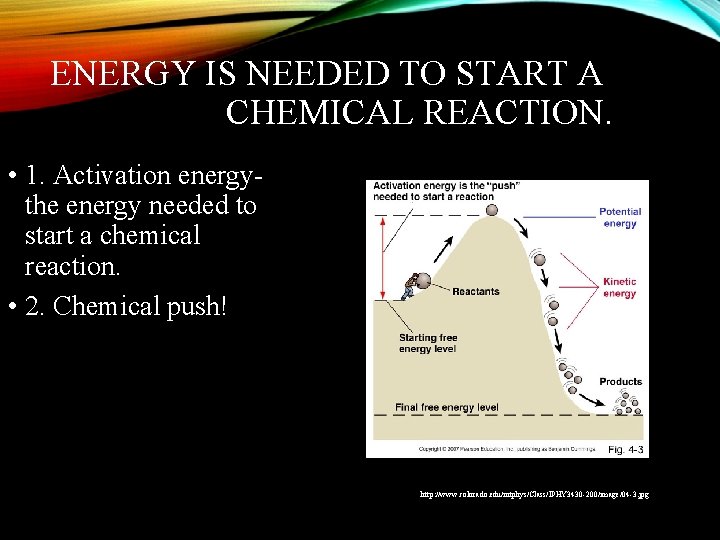
ENERGY IS NEEDED TO START A CHEMICAL REACTION. • 1. Activation energythe energy needed to start a chemical reaction. • 2. Chemical push! http: //www. colorado. edu/intphys/Class/IPHY 3430 -200/image/04 -3. jpg

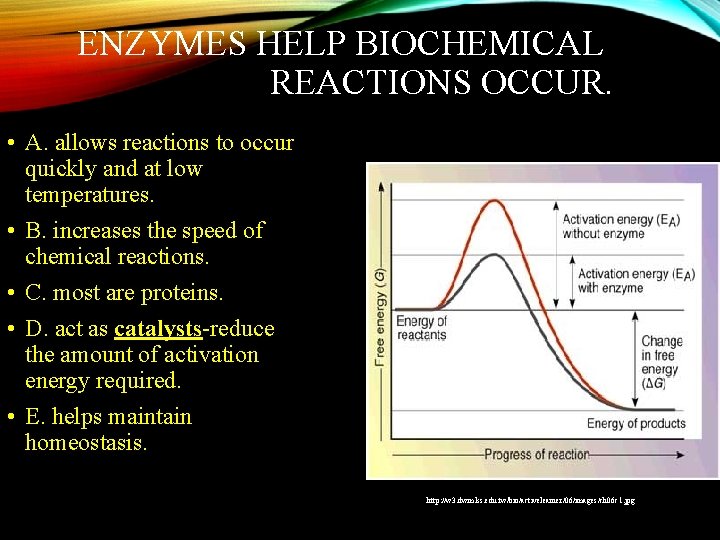
ENZYMES HELP BIOCHEMICAL REACTIONS OCCUR. • A. allows reactions to occur quickly and at low temperatures. • B. increases the speed of chemical reactions. • C. most are proteins. • D. act as catalysts-reduce the amount of activation energy required. • E. helps maintain homeostasis. http: //w 3. dwm. ks. edu. tw/bio/activelearner/06/images/ch 06 c 1. jpg

THREE THINGS THAT EFFECT ENZYME ACTION. • 1. amount of enzyme concentration • 2. Temperature • 3. p. H

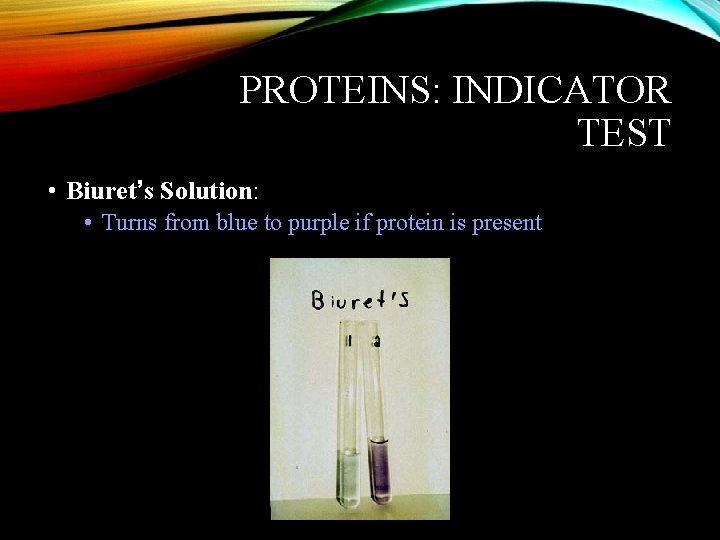
PROTEINS: INDICATOR TEST • Biuret’s Solution: • Turns from blue to purple if protein is present

NUCLEIC ACIDS: FUNCTION • Stores and carries genetic information

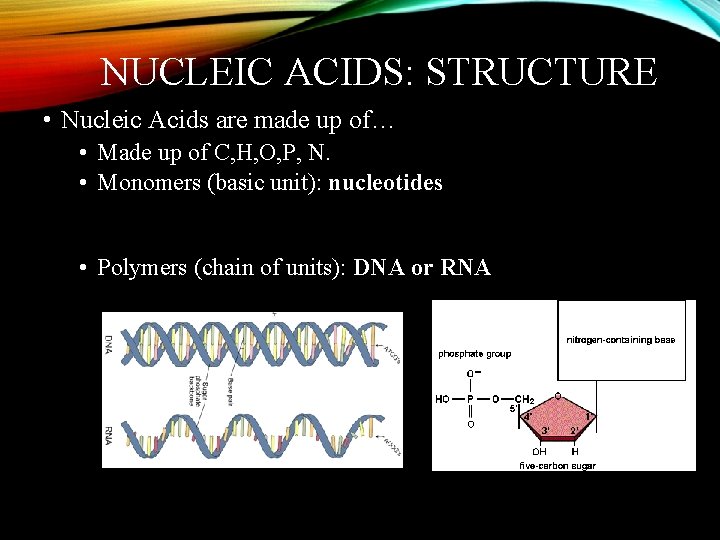
NUCLEIC ACIDS: STRUCTURE • Nucleic Acids are made up of… • Made up of C, H, O, P, N. • Monomers (basic unit): nucleotides • Polymers (chain of units): DNA or RNA

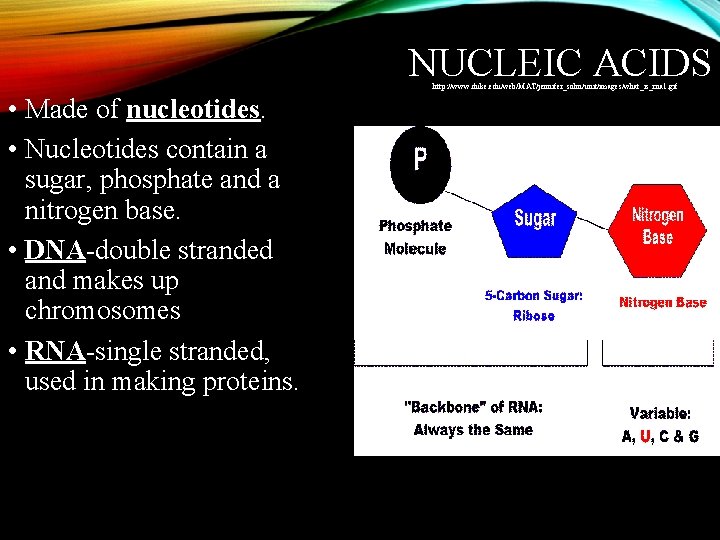
NUCLEIC ACIDS http: //www. duke. edu/web/MAT/jennifer_sohn/unit/images/what_is_rna 1. gif • Made of nucleotides. • Nucleotides contain a sugar, phosphate and a nitrogen base. • DNA-double stranded and makes up chromosomes • RNA-single stranded, used in making proteins.

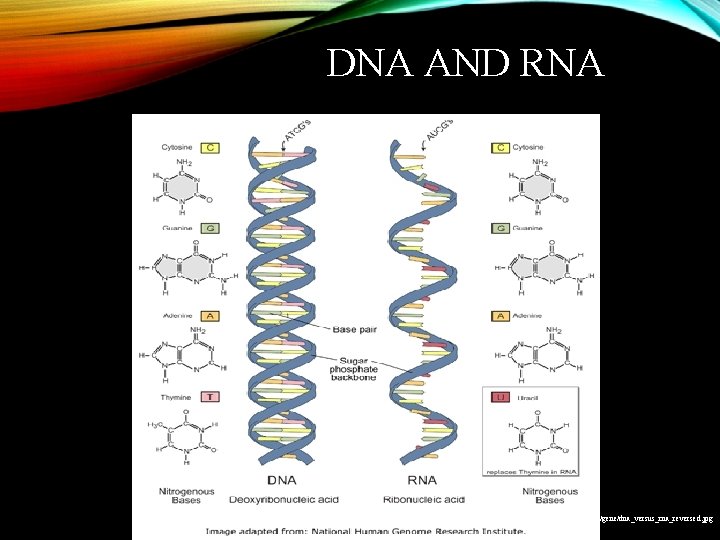
DNA AND RNA http: //images 2. clinicaltools. com/images/gene/dna_versus_rna_reversed. jpg

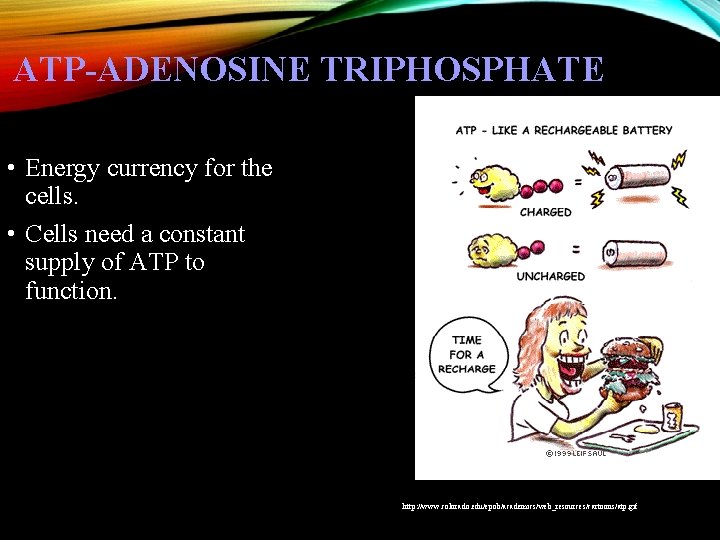
ATP-ADENOSINE TRIPHOSPHATE • Energy currency for the cells. • Cells need a constant supply of ATP to function. http: //www. colorado. edu/epob/academics/web_resources/cartoons/atp. gif

ORGANISMS NEED ENERGY FOR LIFE PROCESSES • Energy- the ability to move or change matter. • A. Energy is stored and released by chemical reactions. • B. Reactants and products • Chemical reaction absorb and release energy • 1. Freezing water releases energy • 2. Melting ice absorbs energy http: //www. windows. ucar. edu/teacher_resources/activities_3 x 3. jpeg

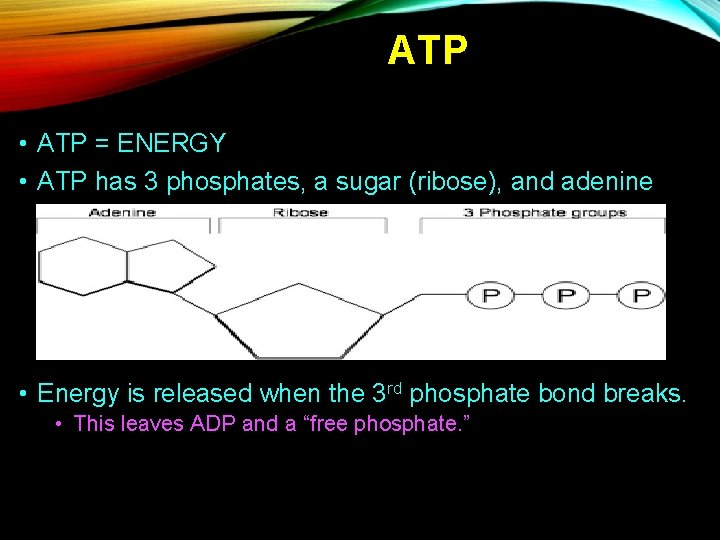
ATP • ATP = ENERGY • ATP has 3 phosphates, a sugar (ribose), and adenine • Energy is released when the 3 rd phosphate bond breaks. • This leaves ADP and a “free phosphate. ”


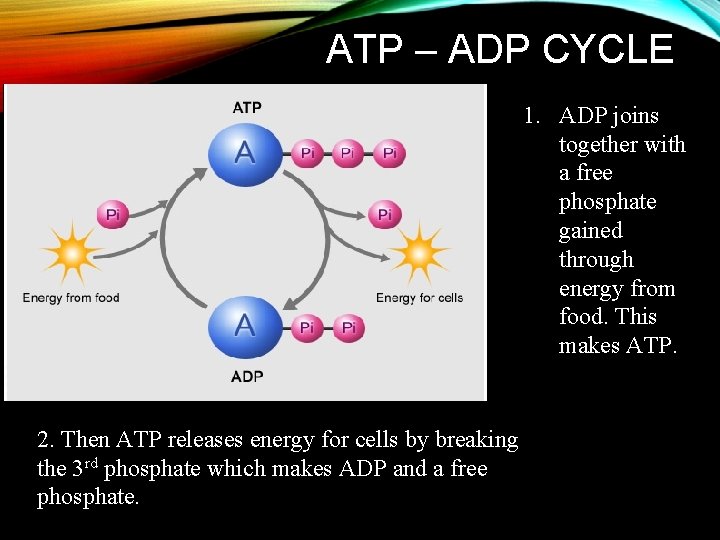
ATP – ADP CYCLE 1. ADP joins together with a free phosphate gained through energy from food. This makes ATP. 2. Then ATP releases energy for cells by breaking the 3 rd phosphate which makes ADP and a free phosphate.
 Chem ufl
Chem ufl Ufl word mat
Ufl word mat Unità foraggere latte
Unità foraggere latte Uf isis
Uf isis How to use uf vpn
How to use uf vpn Gatorperks
Gatorperks Sobha jaishankar
Sobha jaishankar Ib organic chemistry
Ib organic chemistry Inorganic chemistry vs organic chemistry
Inorganic chemistry vs organic chemistry Http //mbs.meb.gov.tr/ http //www.alantercihleri.com
Http //mbs.meb.gov.tr/ http //www.alantercihleri.com Siat.ung.ac.id krs
Siat.ung.ac.id krs Hình ảnh bộ gõ cơ thể búng tay
Hình ảnh bộ gõ cơ thể búng tay Lp html
Lp html Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Chó sói
Chó sói Tư thế worm breton
Tư thế worm breton Chúa yêu trần thế
Chúa yêu trần thế Các môn thể thao bắt đầu bằng tiếng chạy
Các môn thể thao bắt đầu bằng tiếng chạy Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Công của trọng lực
Công của trọng lực Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Cách giải mật thư tọa độ
Cách giải mật thư tọa độ 101012 bằng
101012 bằng Phản ứng thế ankan
Phản ứng thế ankan Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Thể thơ truyền thống
Thể thơ truyền thống Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Một số thể thơ truyền thống
Một số thể thơ truyền thống Bàn tay mà dây bẩn
Bàn tay mà dây bẩn Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Nguyên nhân của sự mỏi cơ sinh 8
Nguyên nhân của sự mỏi cơ sinh 8 đặc điểm cơ thể của người tối cổ
đặc điểm cơ thể của người tối cổ Thứ tự các dấu thăng giáng ở hóa biểu
Thứ tự các dấu thăng giáng ở hóa biểu Vẽ hình chiếu đứng bằng cạnh của vật thể
Vẽ hình chiếu đứng bằng cạnh của vật thể Fecboak
Fecboak Thẻ vin
Thẻ vin đại từ thay thế
đại từ thay thế điện thế nghỉ
điện thế nghỉ Tư thế ngồi viết
Tư thế ngồi viết Diễn thế sinh thái là
Diễn thế sinh thái là Dạng đột biến một nhiễm là
Dạng đột biến một nhiễm là Các số nguyên tố là gì
Các số nguyên tố là gì Tư thế ngồi viết
Tư thế ngồi viết Lời thề hippocrates
Lời thề hippocrates Thiếu nhi thế giới liên hoan
Thiếu nhi thế giới liên hoan ưu thế lai là gì
ưu thế lai là gì Khi nào hổ mẹ dạy hổ con săn mồi
Khi nào hổ mẹ dạy hổ con săn mồi Khi nào hổ mẹ dạy hổ con săn mồi
Khi nào hổ mẹ dạy hổ con săn mồi Hệ hô hấp
Hệ hô hấp Từ ngữ thể hiện lòng nhân hậu
Từ ngữ thể hiện lòng nhân hậu Thế nào là mạng điện lắp đặt kiểu nổi
Thế nào là mạng điện lắp đặt kiểu nổi Iannonechem
Iannonechem Ap chem unit 7
Ap chem unit 7 Types of intermolecular forces
Types of intermolecular forces Chem 130 final exam
Chem 130 final exam Met et prop but
Met et prop but Principles of kmt
Principles of kmt January 25 2018 chemistry regents answers
January 25 2018 chemistry regents answers Beer's law equation example
Beer's law equation example Chem 1020
Chem 1020 Chem law hazard pay
Chem law hazard pay Gen chem review for ochem
Gen chem review for ochem Chem 109
Chem 109 Chem feed
Chem feed Chem portal
Chem portal Chem 260 umich
Chem 260 umich Chem pro 100
Chem pro 100 Chem 253
Chem 253 Chapter 19 acids bases and salts worksheet answers
Chapter 19 acids bases and salts worksheet answers Ka and kb
Ka and kb Thermochemistry vocabulary
Thermochemistry vocabulary Magnesium pes spectrum
Magnesium pes spectrum