Chem 253 128 Lecture Introduction Instructors Roy Dixon













- Slides: 31

Chem. 253 – 1/28 Lecture

Introduction - Instructors: Roy Dixon • Educational Background in Environmental Analytical Chemistry • My research in environmental chemistry has been in the following areas: • Cloud and Precipitation Chemistry • Measurement of constituents of cloud droplets, rain and snow • Effects of snow formation type on chemical composition • Aerosol Composition and Tracer Measurement • Bio- and Synthetic Fuel Testing

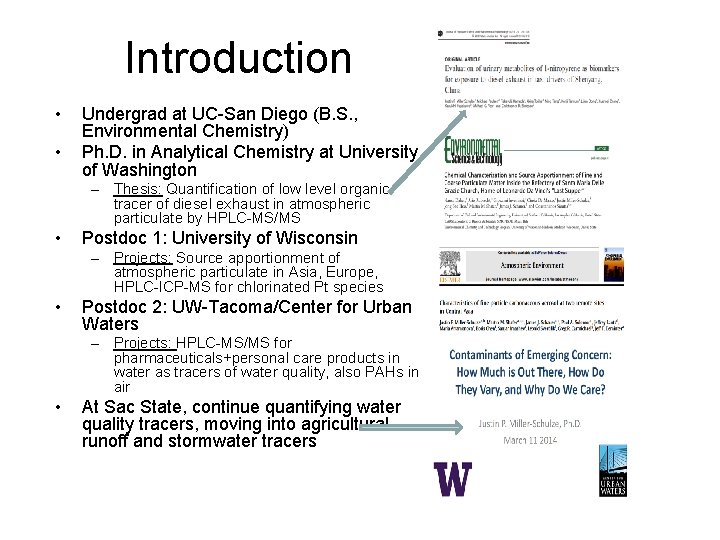
Introduction • • Undergrad at UC-San Diego (B. S. , Environmental Chemistry) Ph. D. in Analytical Chemistry at University of Washington – Thesis: Quantification of low level organic tracer of diesel exhaust in atmospheric particulate by HPLC-MS/MS • Postdoc 1: University of Wisconsin – Projects: Source apportionment of atmospheric particulate in Asia, Europe, HPLC-ICP-MS for chlorinated Pt species • Postdoc 2: UW-Tacoma/Center for Urban Waters – Projects: HPLC-MS/MS for pharmaceuticals+personal care products in water as tracers of water quality, also PAHs in air • At Sac State, continue quantifying water quality tracers, moving into agricultural runoff and stormwater tracers

Introduction - Students • Introduce yourself (name/degree plan) • Is there anything specific you expect to get out of the class?

Syllabus – Instructors • I will be teaching the first third (atmospheric chemistry) and last third (various topics) and Dr. Miller-Schulze will be covering the middle third (water chemistry and various compound classes) • I will maintain a website through my individual website/Dr. Miller-Schulze will use Blackboard

Syllabus – Meeting/Exams • In graduate classes, I will typically have a 10 minute break in the middle of the lecture (with class ending at 8: 10 instead) • This may be skipped due to class assignment in middle of class period • Exams: 3 exams (one on each third – no cumulative exam; exam 3 is on day of final but will be as long as exams 1 and 2) • Exams will involve qualitative and quantitative knowledge

Syllabus – Text/Exams/Reading • Because of the single 2. 5 block of time, we will try to have group activities in the middle of the lecture • Exams: 3 exams (one 1 hour on each third – no cumulative exam; exam 3 is on day of final) • Exams will involve qualitative and quantitative knowledge • Most of the information will come from the Baird and Cann text (with some supplementary readings made available)

Syllabus - Course Overview • The course is partially broken up by “spheres” – – – Atmosphere Hydrosphere Lithosphere Others: Biosphere We study chemistry in each regime • Environmental Chemists can also study: – Natural (unperturbed) Systems – Cause and Effect of Perturbations (e. g. pollution) – Ways to Mitigate or Adapt to Perturbation

Syllabus - Notes on Grading I • Exams: 84% of total (28% each) • Homework (8% of total) – Mostly assigned from book – Some problems (e. g. review questions) are not graded – Will randomly select one or two problems for grading – must show work, not just answer

Syllabus - Notes on Grading II • In Class Group Assignments (8% of grade) - Goal is to increase in-class participation, break up a long lecture, and encourage learning by doing - Expect to have groups of 3 (could depend on class size), assigned by instructor, with different tasks assigned to each participant - Will work on problems, and then turn in results - New activity for me - Will start with an ungraded activity today

Syllabus - Notes on Grading III • Group Assignments (cont. ) • This is how the active learning exercises will work logistically: – There will be a class list available that gives the groups and roles for each worksheet – You’ll sit with your group (by number) and work on an Activity • Groups will be of 3 (and some 2’s or 4’s depending on attendance, # of students in class) – One person in the group will be “the calculator”, one person will be “the recorder”, one person will be “the manager”

Syllabus - Notes on Grading IV • Group Assignments (cont. ) • Role Descriptions: – Calculator (C): This is the person who operates the calculator and performs all calculations – Recorder (R): This is the only person who can write answers on the worksheet to be turned in at the end – Manager (M): This is the person who manages the group. This is the only person who can ask me questions during the “active learning” time

Typical Lectures (My part) • Mostly by powerpoint (with some example calculations) • Announcements in beginning • Powerpoint slides will be made available on website • Group activities will be in the middle (~30 min) • No break planned, but I could have a break before or following the group activity

Homework Set 1 • To do before 1 st Exam. • Working on entire set, but • Set 1. 1: Ch. 1 • • Problems: 2, 4, 5 Review Questions: 1, 3 -10 Additional Problems: 1, 5 bold problems to be turned in next lecture • Problems to be turned in should be worked on independently.

Today’s Topics • • Biogeochemical Cycles Introduction to Atmospheric Chemistry The Stratospheric Chemistry

Biogeochemical Cycles • Mostly not covered in text • What is a Biogeochemical Cycle? – If we can define different regions as “spheres”, we can define biogeochemical cycles as the set of spheres and the flow paths in and between different spheres • Why am I covering them? – They are very useful for putting problems in perspective – Examples: • anthropogenic vs. natural sources • reservoir vs. flux species

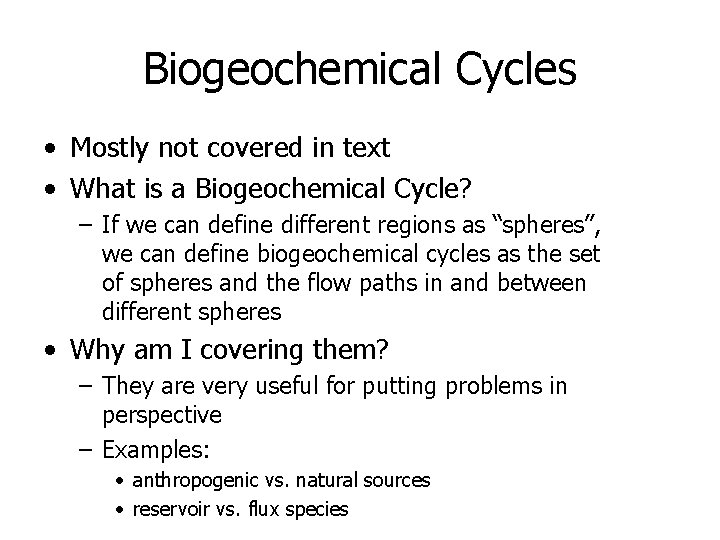
Biogeochemical Cycles • Familiar Example (that is in text) Water Cycle – a largely non-chemical transformation cycle Green Numbers are Amounts (Reservoirs) Black Numbers are Fluxes (transport from one to another reservoir) Baird and Cann, p. 410

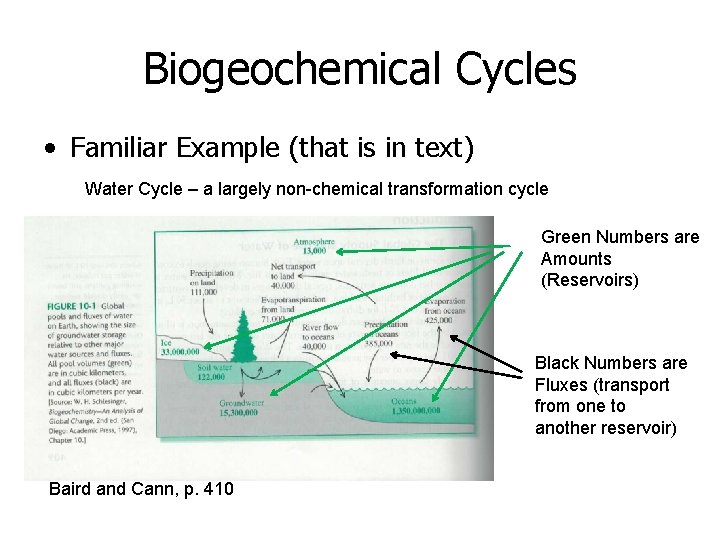
Biogeochemical Cycles • Can also use box and arrow approach • “Box models” are among the simplest models describing cycling atmosphere Ice oceans Note: atmosphere is nearly insignificant as reservoir, but very important for fluxes

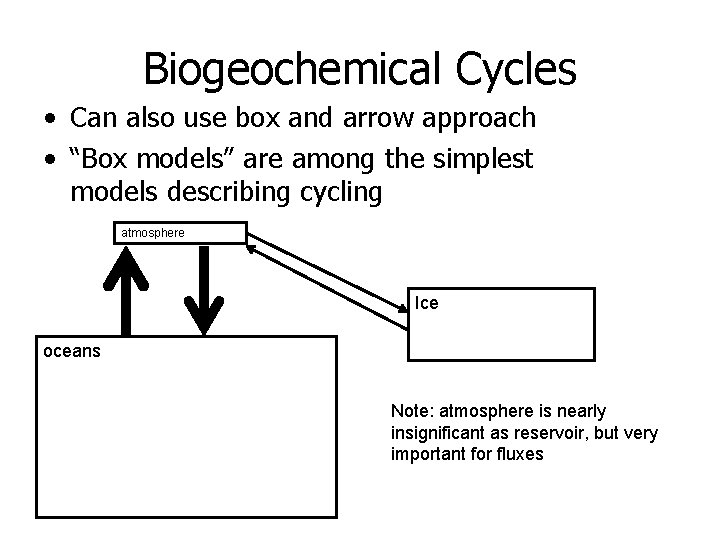
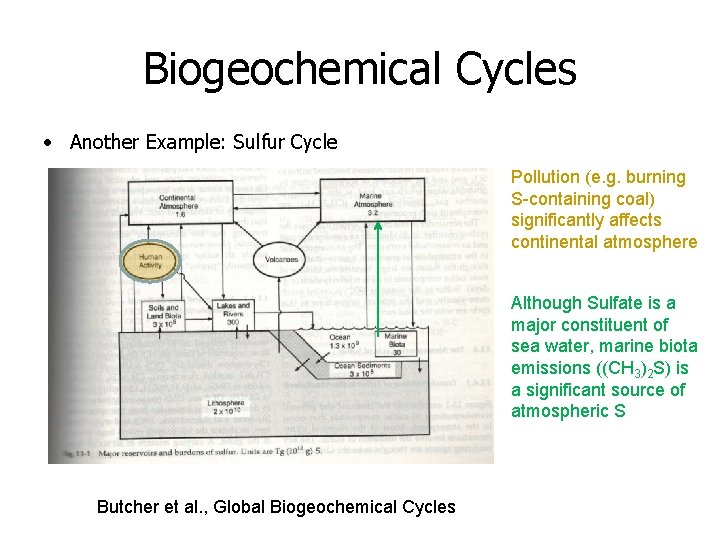
Biogeochemical Cycles • Another Example: Sulfur Cycle Pollution (e. g. burning S-containing coal) significantly affects continental atmosphere Although Sulfate is a major constituent of sea water, marine biota emissions ((CH 3)2 S) is a significant source of atmospheric S Butcher et al. , Global Biogeochemical Cycles

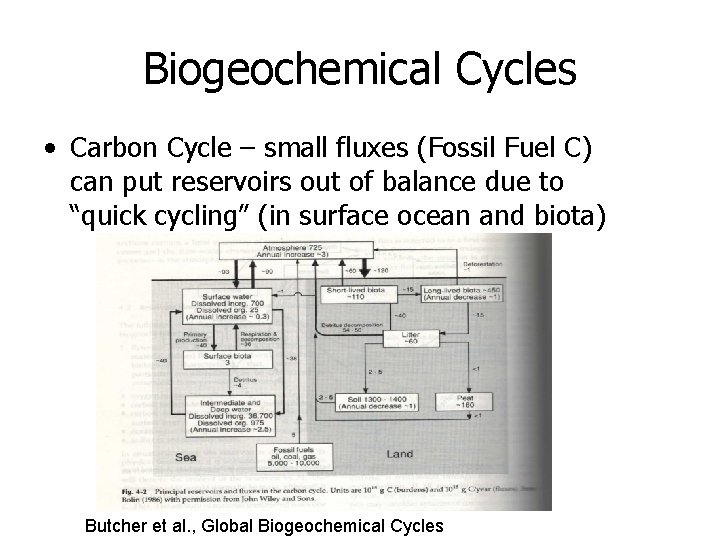
Biogeochemical Cycles • Carbon Cycle – small fluxes (Fossil Fuel C) can put reservoirs out of balance due to “quick cycling” (in surface ocean and biota) Butcher et al. , Global Biogeochemical Cycles

Biogeochemical Cycles • Cycles covered are very general and don’t cover a variety of pathways within boxes • Additionally most spheres or boxes need to be subdivided due to differences in pathways Butcher et al. , Global Biogeochemical Cycles

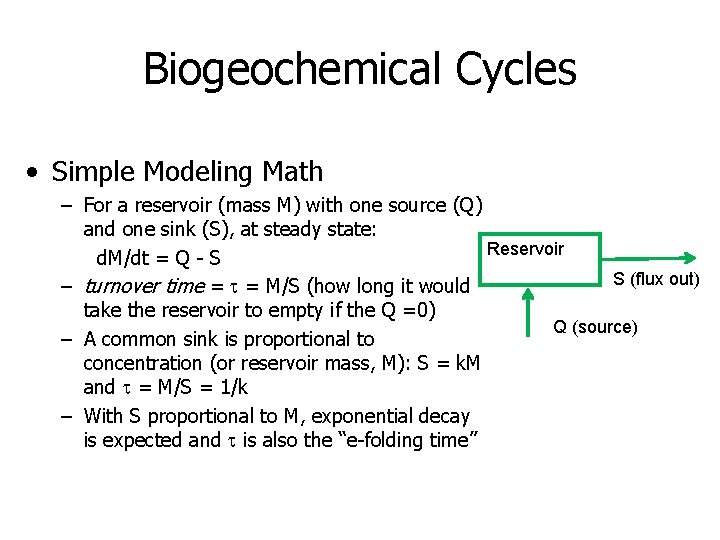
Biogeochemical Cycles • Simple Modeling Math – For a reservoir (mass M) with one source (Q) and one sink (S), at steady state: Reservoir d. M/dt = Q - S S (flux out) – turnover time = t = M/S (how long it would take the reservoir to empty if the Q =0) Q (source) – A common sink is proportional to concentration (or reservoir mass, M): S = k. M and t = M/S = 1/k – With S proportional to M, exponential decay is expected and t is also the “e-folding time”

Introduction to Atmospheric Chemistry • Atmospheric Composition – 78% nitrogen (N 2) – pretty inert – 21% oxygen (O 2) – fairly inert in lower atmosphere – ~1% Ar • Concentration units (for non-major species) – parts per million by volume (ppmv) = (n. X/nair)· 106 (where n = moles) – partial pressures (atm, e. g. for Henry’s law calculations) – concentrations (molecules/cm 3 for kinetic calculations) – A well mixed gas (e. g. Ar) will have constant mixing ratio with altitude but a decreasing concentration

Introduction to Atmospheric Chemistry • Source of Oxygen – Not a stable gas (metals, carbon like to be oxidized) – Produced by biota (and then changed the atmosphere) • Structure of Atmosphere – Pressure decreases with height (as with other fluids) – If the atmosphere is considered isothermal (it isn’t), P = Poe-Z/H (where P = pressure, Po = 1 atm, Z = height, and H ~ 8 km)

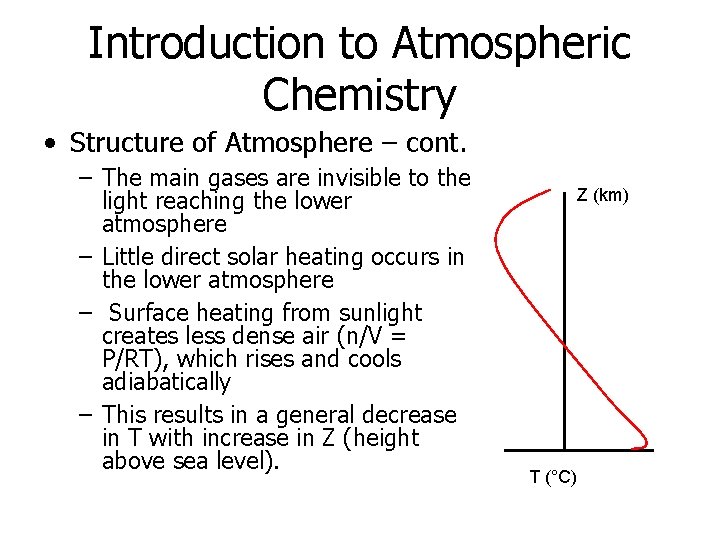
Introduction to Atmospheric Chemistry • Structure of Atmosphere – cont. – The main gases are invisible to the light reaching the lower atmosphere – Little direct solar heating occurs in the lower atmosphere – Surface heating from sunlight creates less dense air (n/V = P/RT), which rises and cools adiabatically – This results in a general decrease in T with increase in Z (height above sea level). Z (km) T (°C)

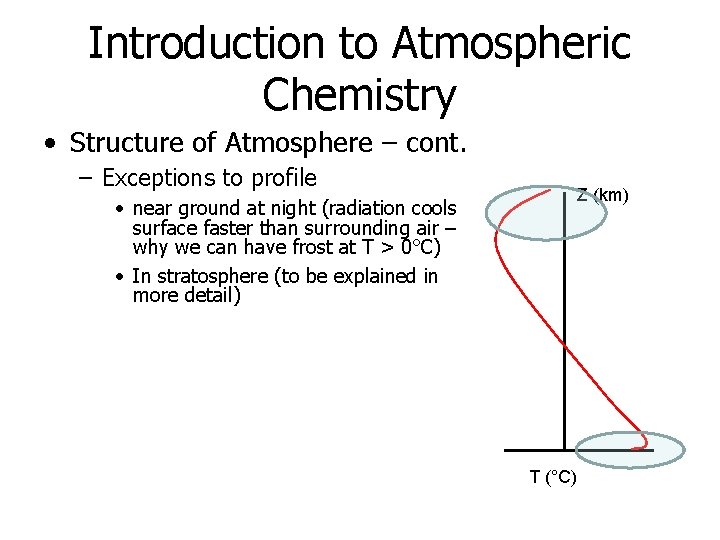
Introduction to Atmospheric Chemistry • Structure of Atmosphere – cont. – Exceptions to profile • near ground at night (radiation cools surface faster than surrounding air – why we can have frost at T > 0°C) • In stratosphere (to be explained in more detail) Z (km) T (°C)

Introduction to Atmospheric Chemistry • Atmospheric Layers – highest layers (not covered here) – stratosphere (~12 to 48 km) – troposphere (0 to ~12 km) • Stratosphere occurs due to change in lapse rate due to atmospheric heating from absorption of solar light • Warmer stratosphere makes mixing with lower atmosphere very slow (hard to get cold tropospheric air to rise into warmer stratosphere)

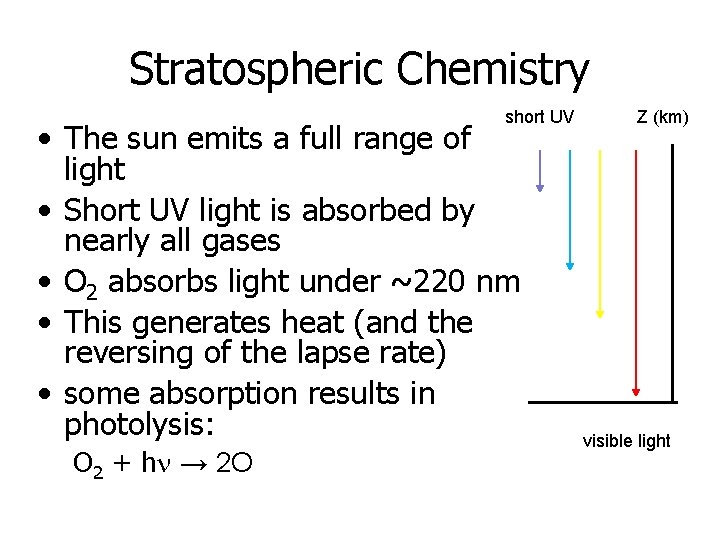
Stratospheric Chemistry short UV • The sun emits a full range of light • Short UV light is absorbed by nearly all gases • O 2 absorbs light under ~220 nm • This generates heat (and the reversing of the lapse rate) • some absorption results in photolysis: O 2 + hn → 2 O Z (km) visible light

Stratospheric Chemistry • Prediction of efficacy of light for photolyzing bonds: – can compare Ephoton with Ebond – when Ephoton > Ebond, photolysis is possible – Ephoton = hc/l (note: calculated per molecule while E is given per mole)

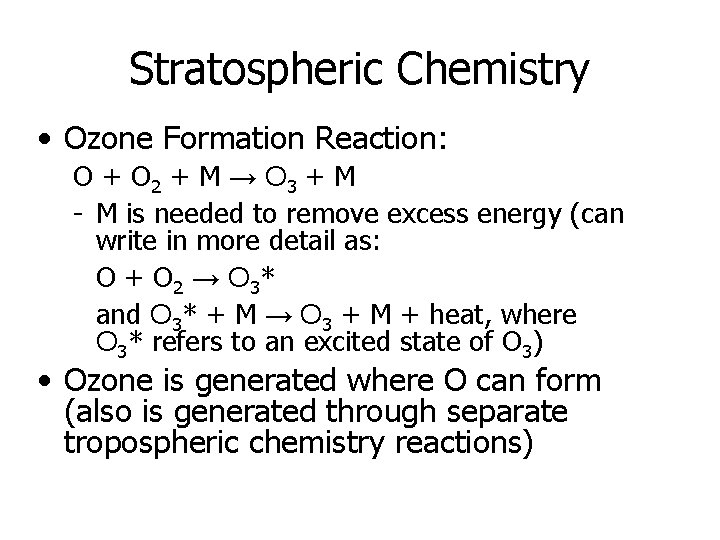
Stratospheric Chemistry • Ozone Formation Reaction: O + O 2 + M → O 3 + M - M is needed to remove excess energy (can write in more detail as: O + O 2 → O 3* and O 3* + M → O 3 + M + heat, where O 3* refers to an excited state of O 3) • Ozone is generated where O can form (also is generated through separate tropospheric chemistry reactions)

Stratospheric Chemistry • Value of Ozone in the Stratosphere – Ozone has weaker bonds than O 2 and absorbs longer wavelength UV light – Absorbs light effectively in the 220 to 290 nm range – This protects life in the lower atmosphere • Full Set of O only reactions O 3 + hn → O 2* + O* and O 3 + O → 2 O 2 (odd O ending rxn)