AP Chemistry Unit 2 2 Photoelectron Spectroscopy Day






- Slides: 28

AP Chemistry Unit 2. 2 – Photoelectron Spectroscopy Day 1: Photoelectron Spectroscopy, Photoelectric effect, Photoelectron Spectroscopy Practice

Warm Up TAKE OUT: Structure of AP Exam handout (given last class) SKIM THROUGH: Information HIGHLIGHT: Areas where you have questions TIME: 4 MINUTES WHEN DONE: Share out with your table partners what you highlighted

Agenda • Notes: Photoelectron Spectroscopy and Ionization Energy • Demonstration: Electroscope • Practice: Reading a PES Spectrum

PES Instrument Image Source: SPECS Gmb. H, http: //www. specs. de/cms/front_content. php? idart=267

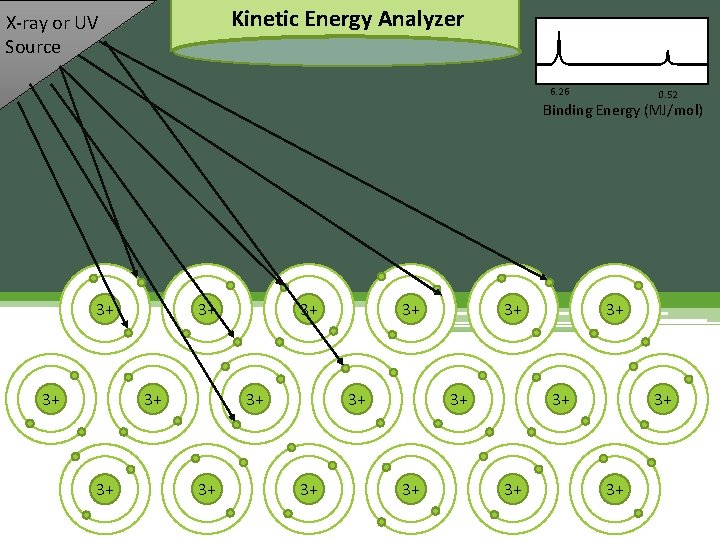
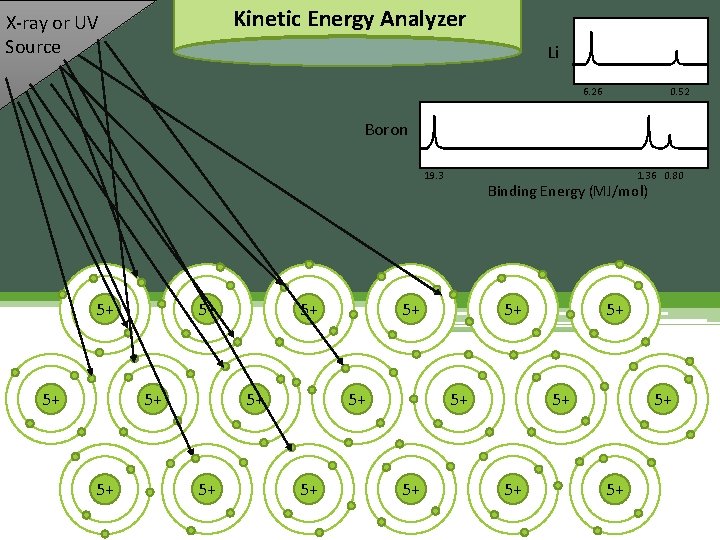
Kinetic Energy Analyzer X-ray or UV Source 6. 26 0. 52 Binding Energy (MJ/mol) 3+ 3+ 3+ 3+ 3+

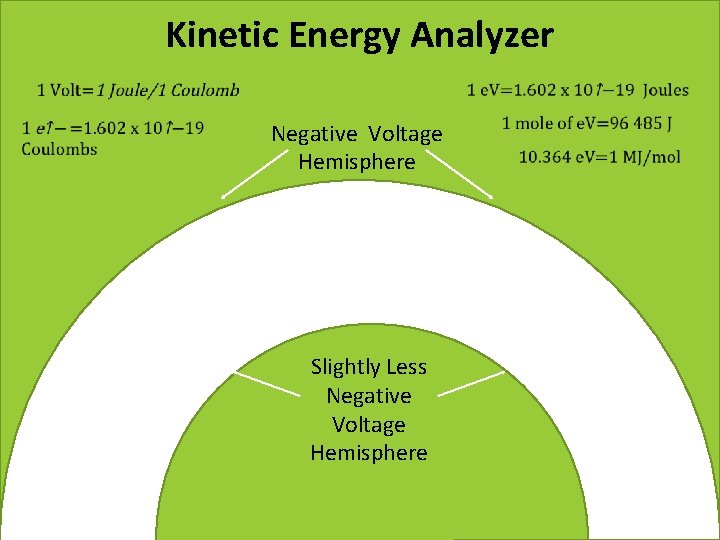
Kinetic Energy Analyzer Negative Voltage Hemisphere Slightly Less Negative Voltage Hemisphere

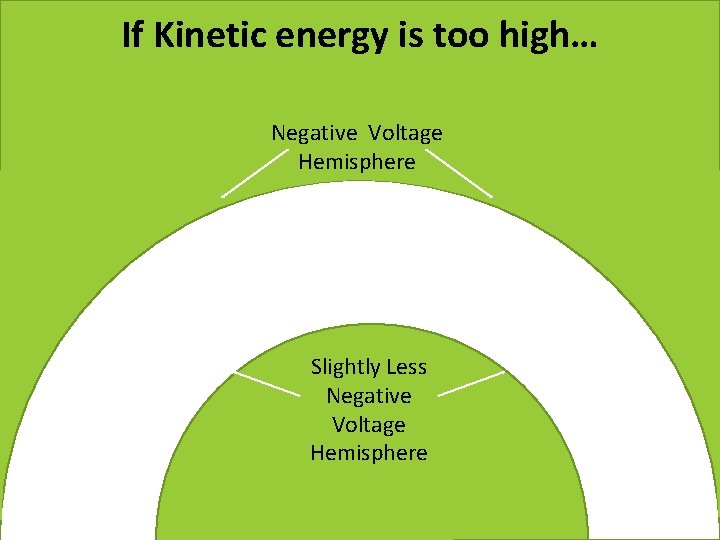
If Kinetic energy is too high… Negative Voltage Hemisphere Slightly Less Positive Negative Voltage Hemisphere

If voltage is too high… Negative Voltage Hemisphere Slightly Less Positive Negative Voltage Hemisphere

Kinetic Energy Analyzer Kinetic X-ray or UV Source Li 6. 26 Binding Energy (MJ/mol) Boron 1. 36 0. 80 19. 3 5+ 5+ 3+ 3+ 5+ 5+ 3+ 3+ 5+ Binding Energy (MJ/mol) 5+ 3+ 3+ 5+ 0. 52 5+ 3+ 3+ 5+ 5+ 3+

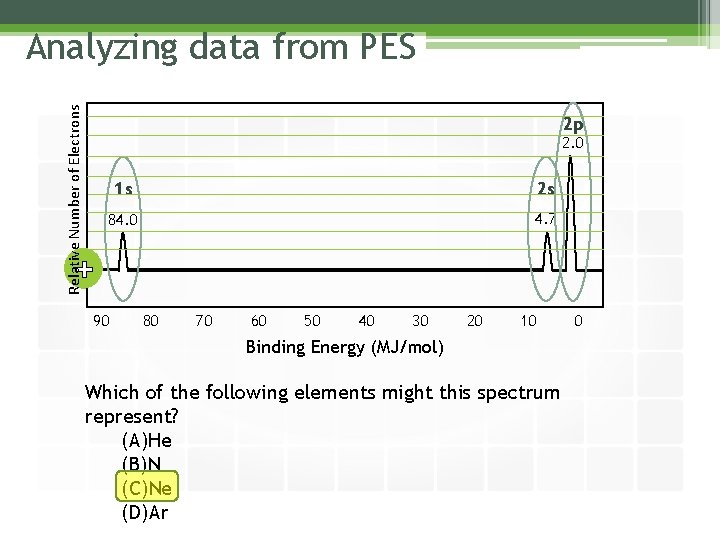
Relative Number of Electrons Analyzing data from PES 2 p 2. 0 1 s 2 s 84. 0 4. 7 + 90 80 70 60 50 40 30 20 10 Binding Energy (MJ/mol) Which of the following elements might this spectrum represent? (A)He (B)N (C)Ne (D)Ar 0

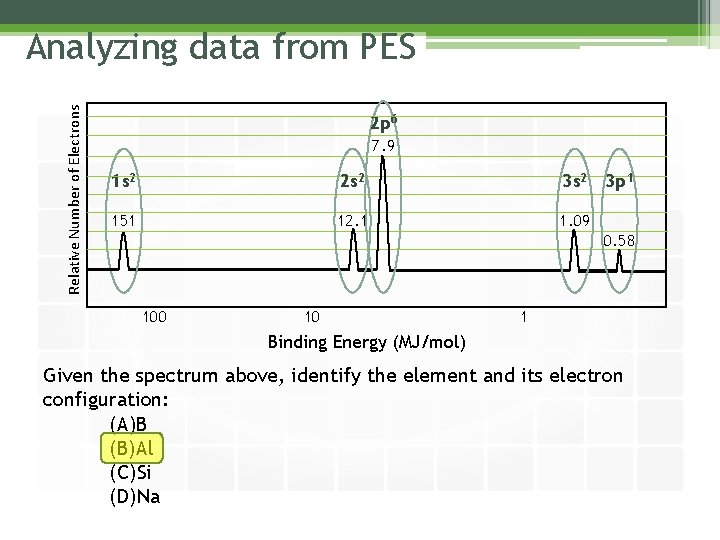
Relative Number of Electrons Analyzing data from PES 2 p 6 7. 9 1 s 2 2 s 2 3 s 2 151 12. 1 1. 09 3 p 1 0. 58 100 10 1 Binding Energy (MJ/mol) Given the spectrum above, identify the element and its electron configuration: (A)B (B)Al (C)Si (D)Na

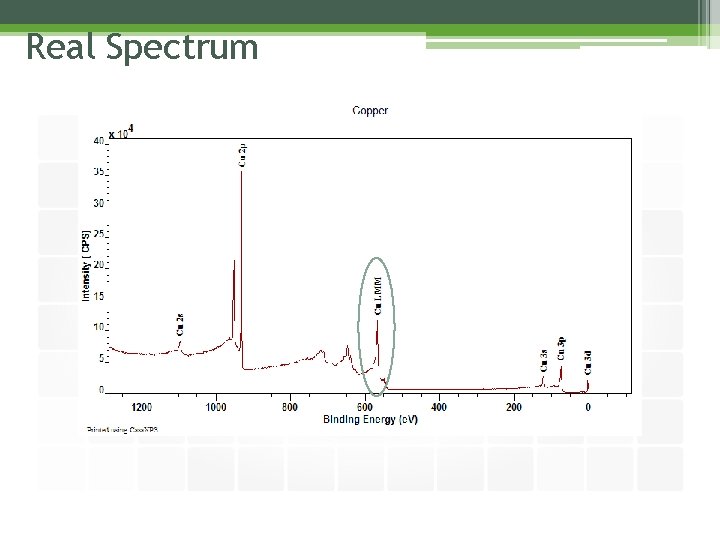
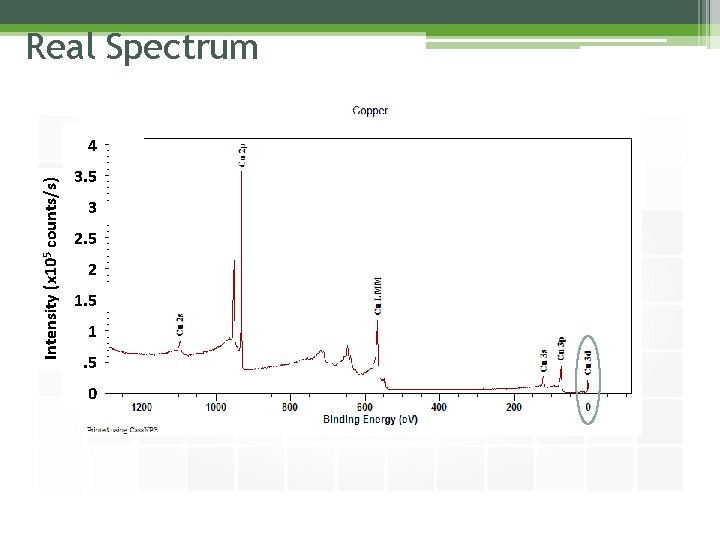
Real Spectrum

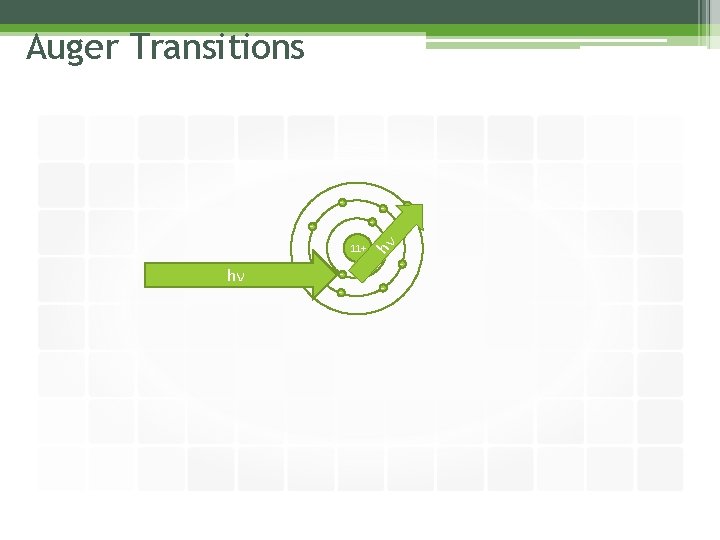
Auger Transitions 11+ hν - - -

Real Spectrum Intensity (x 105 counts/s) 4 3. 5 3 2. 5 2 1. 5 0

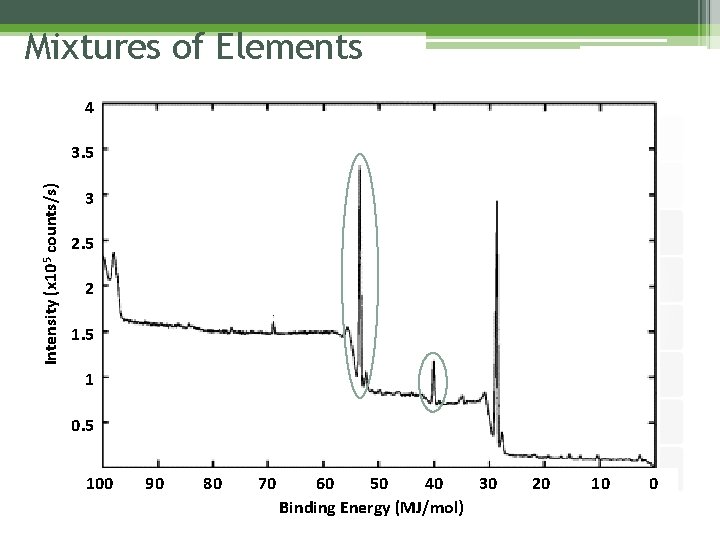
Mixtures of Elements 4 Intensity (x 105 counts/s) 3. 5 3 2. 5 2 1. 5 1 0. 5 100 90 80 70 60 50 40 30 Binding Energy (MJ/mol) 20 10 0

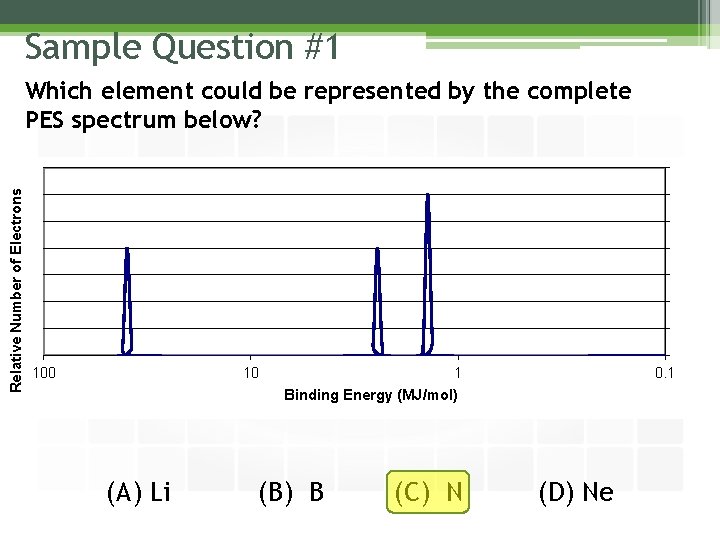
Sample Question #1 Relative Number of Electrons Which element could be represented by the complete PES spectrum below? 100 10 (A) Li 1 Binding Energy (MJ/mol) (B) B (C) N 0. 1 (D) Ne

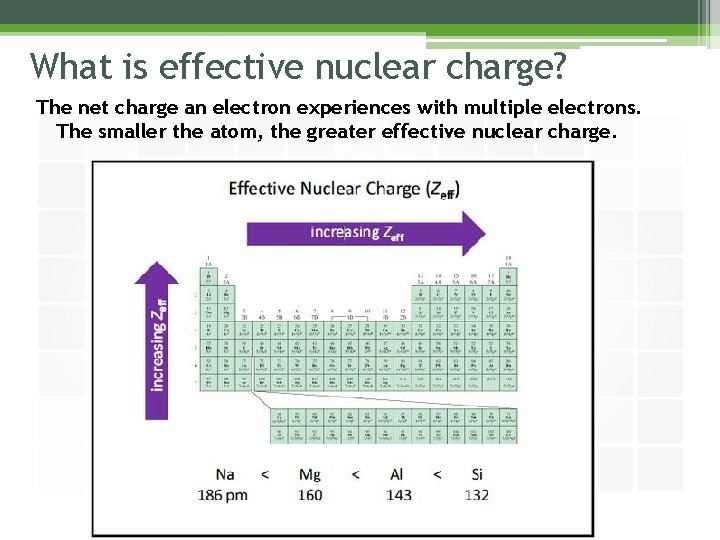
What is effective nuclear charge? The net charge an electron experiences with multiple electrons. The smaller the atom, the greater effective nuclear charge.

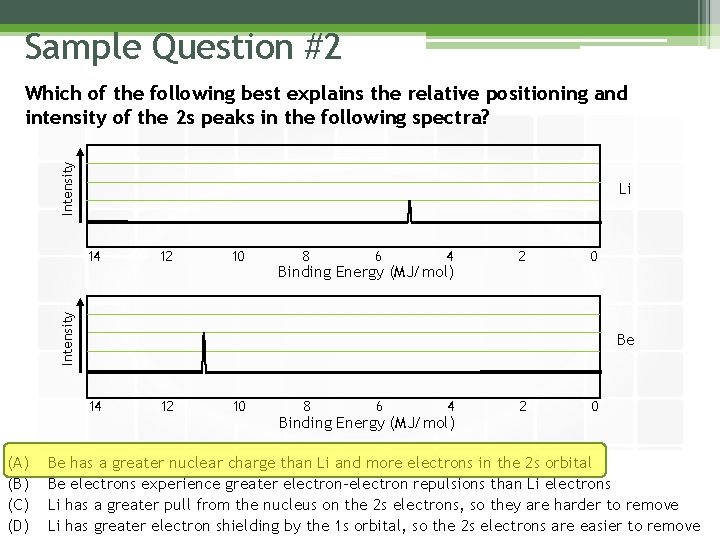
Sample Question #2 Intensity Which of the following best explains the relative positioning and intensity of the 2 s peaks in the following spectra? Li 12 10 8 6 4 Binding Energy (MJ/mol) 2 0 Intensity 14 Be 14 (A) (B) (C) (D) 12 10 8 6 4 Binding Energy (MJ/mol) 2 0 Be has a greater nuclear charge than Li and more electrons in the 2 s orbital Be electrons experience greater electron-electron repulsions than Li electrons Li has a greater pull from the nucleus on the 2 s electrons, so they are harder to remove Li has greater electron shielding by the 1 s orbital, so the 2 s electrons are easier to remove

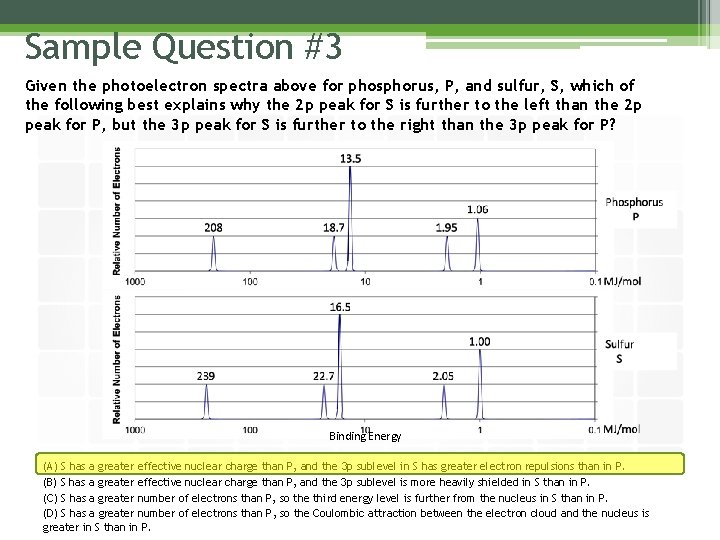
Sample Question #3 Given the photoelectron spectra above for phosphorus, P, and sulfur, S, which of the following best explains why the 2 p peak for S is further to the left than the 2 p peak for P, but the 3 p peak for S is further to the right than the 3 p peak for P? Binding Energy (A) S has a greater effective nuclear charge than P, and the 3 p sublevel in S has greater electron repulsions than in P. (B) S has a greater effective nuclear charge than P, and the 3 p sublevel is more heavily shielded in S than in P. (C) S has a greater number of electrons than P, so the third energy level is further from the nucleus in S than in P. (D) S has a greater number of electrons than P, so the Coulombic attraction between the electron cloud and the nucleus is greater in S than in P.

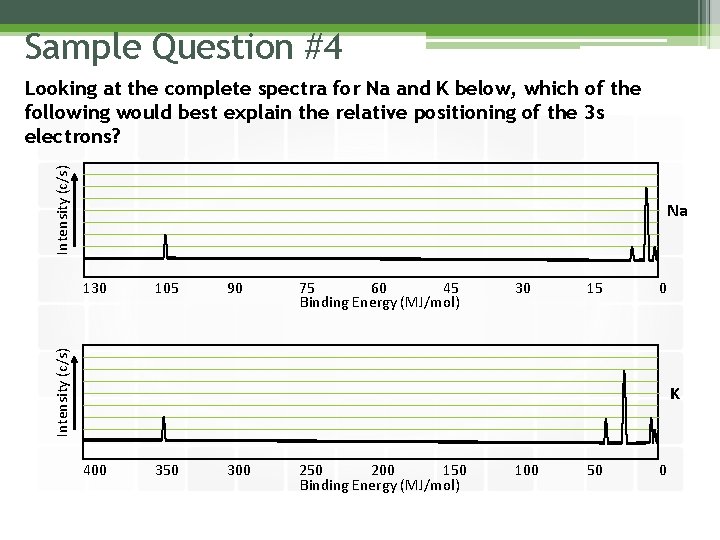
Sample Question #4 Intensity (c/s) Looking at the complete spectra for Na and K below, which of the following would best explain the relative positioning of the 3 s electrons? Na 105 90 75 60 45 Binding Energy (MJ/mol) 30 15 0 Intensity (c/s) 130 K 400 350 300 250 200 150 Binding Energy (MJ/mol) 100 50 0

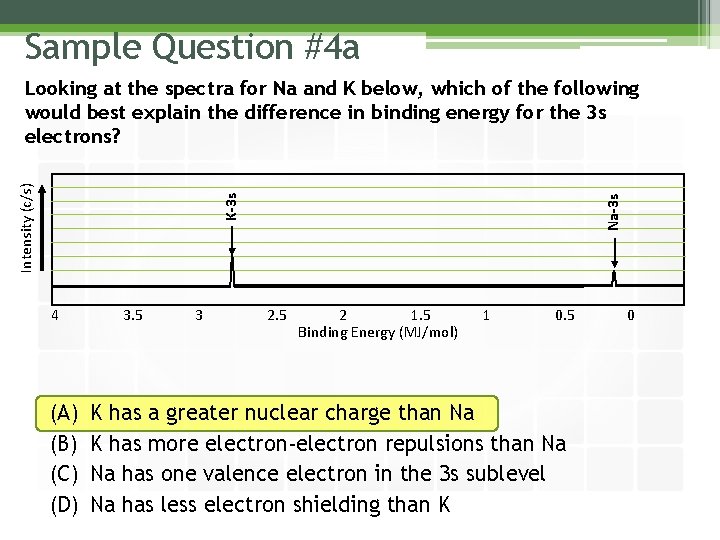
Sample Question #4 a 4 (A) (B) (C) (D) 3. 5 3 Na-3 s K-3 s Intensity (c/s) Looking at the spectra for Na and K below, which of the following would best explain the difference in binding energy for the 3 s electrons? 2. 5 2 1. 5 Binding Energy (MJ/mol) 1 0. 5 K has a greater nuclear charge than Na K has more electron-electron repulsions than Na Na has one valence electron in the 3 s sublevel Na has less electron shielding than K 0

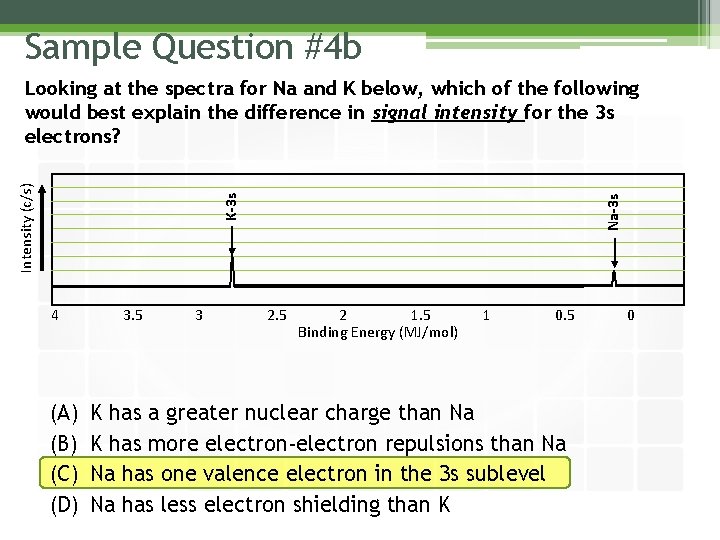
Sample Question #4 b 4 (A) (B) (C) (D) 3. 5 3 Na-3 s K-3 s Intensity (c/s) Looking at the spectra for Na and K below, which of the following would best explain the difference in signal intensity for the 3 s electrons? 2. 5 2 1. 5 Binding Energy (MJ/mol) 1 0. 5 K has a greater nuclear charge than Na K has more electron-electron repulsions than Na Na has one valence electron in the 3 s sublevel Na has less electron shielding than K 0

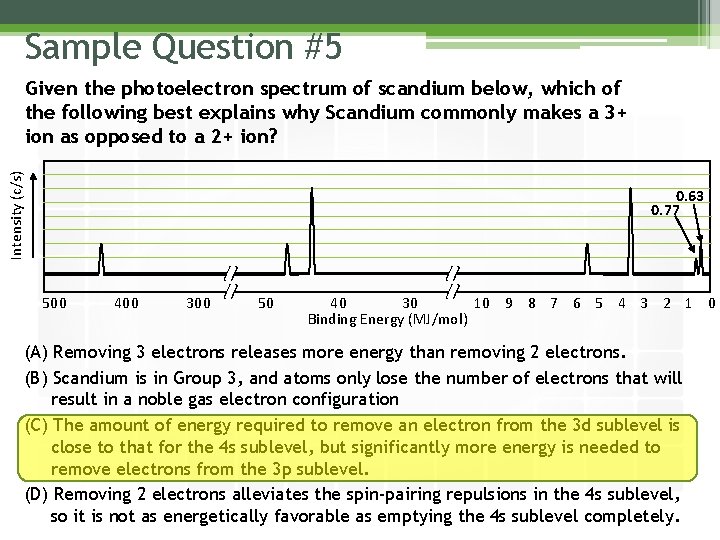
Sample Question #5 Intensity (c/s) Given the photoelectron spectrum of scandium below, which of the following best explains why Scandium commonly makes a 3+ ion as opposed to a 2+ ion? 0. 63 0. 77 500 400 300 50 40 30 10 9 8 7 6 5 4 3 2 1 0 Binding Energy (MJ/mol) (A) Removing 3 electrons releases more energy than removing 2 electrons. (B) Scandium is in Group 3, and atoms only lose the number of electrons that will result in a noble gas electron configuration (C) The amount of energy required to remove an electron from the 3 d sublevel is close to that for the 4 s sublevel, but significantly more energy is needed to remove electrons from the 3 p sublevel. (D) Removing 2 electrons alleviates the spin-pairing repulsions in the 4 s sublevel, so it is not as energetically favorable as emptying the 4 s sublevel completely.

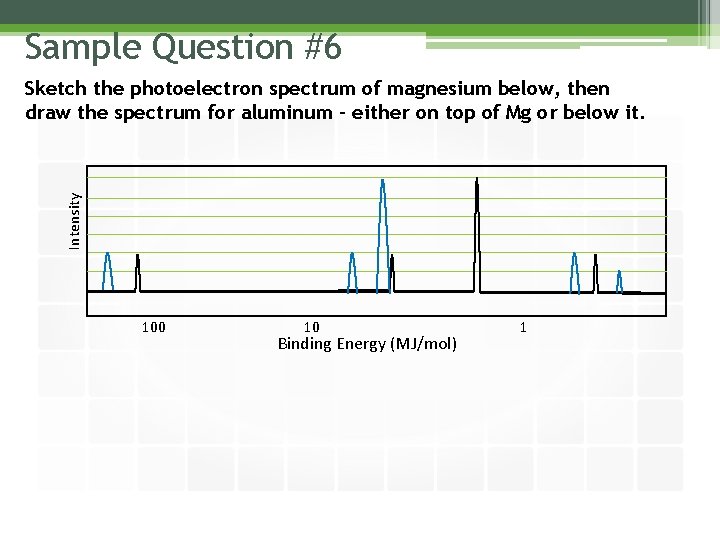
Sample Question #6 Intensity Sketch the photoelectron spectrum of magnesium below, then draw the spectrum for aluminum – either on top of Mg or below it. 100 10 Binding Energy (MJ/mol) 1

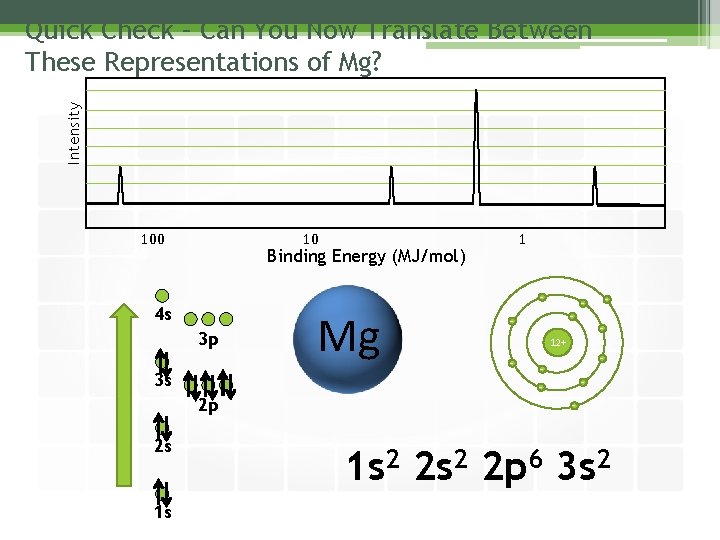
Intensity Quick Check – Can You Now Translate Between These Representations of Mg? 100 10 Binding Energy (MJ/mol) - 4 s 3 p 3 s 2 p 2 s 1 s 1 Mg - - - 12+ - - - 1 s 2 2 p 6 3 s 2

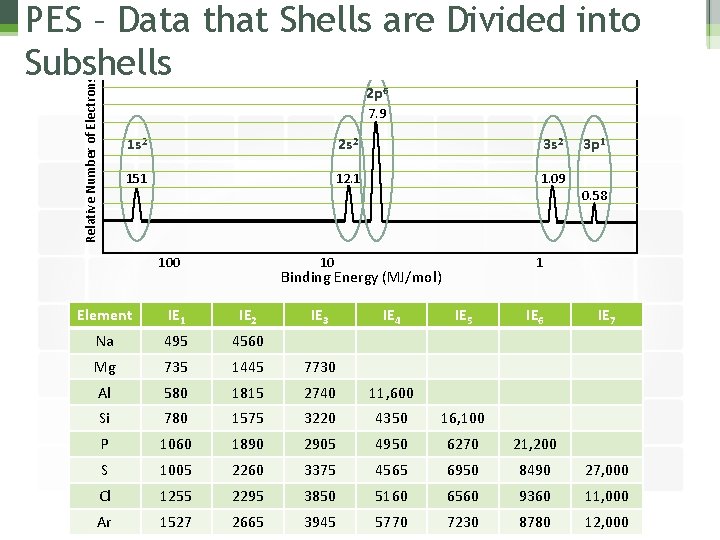
Relative Number of Electrons PES – Data that Shells are Divided into Subshells 2 p 6 7. 9 1 s 2 2 s 2 3 s 2 151 12. 1 1. 09 100 10 IE 4 0. 58 1 Binding Energy (MJ/mol) IE 3 3 p 1 Element IE 1 IE 2 IE 5 IE 6 IE 7 Na 495 4560 Mg 735 1445 7730 Al 580 1815 2740 11, 600 Si 780 1575 3220 4350 16, 100 P 1060 1890 2905 4950 6270 21, 200 S 1005 2260 3375 4565 6950 8490 27, 000 Cl 1255 2295 3850 5160 6560 9360 11, 000 Ar 1527 2665 3945 5770 7230 8780 12, 000

Electroscope demonstration

Photoelectron Spectroscopy WITH TABLE PARTNERS: Work on Photoelectron Spectroscopy handout TIME: Until the end of class WHEN DONE: Look over assignments for next week
 Spectroscopy ap chem
Spectroscopy ap chem Photoelectron spectroscopy ap chemistry
Photoelectron spectroscopy ap chemistry Magnesium pes spectrum
Magnesium pes spectrum Xps instrument
Xps instrument Day 1 day 2 day 3 day 4
Day 1 day 2 day 3 day 4 Day 1 day 2 day 817
Day 1 day 2 day 817 Ib chemistry functional groups
Ib chemistry functional groups Inorganic chemistry vs organic chemistry
Inorganic chemistry vs organic chemistry Unit 6 review questions
Unit 6 review questions William beanes elementary school
William beanes elementary school Ocean the part day after day
Ocean the part day after day Day to day maintenance
Day to day maintenance As your room gets messier day by day, entropy is
As your room gets messier day by day, entropy is Tomorrow i don't know
Tomorrow i don't know Timeline for acts i-iii
Timeline for acts i-iii Growing day by day
Growing day by day Seed germination inhibitors examples
Seed germination inhibitors examples Day by day seed germination observation chart
Day by day seed germination observation chart Geotropism
Geotropism I live for jesus day after day
I live for jesus day after day Glorious day one day when heaven
Glorious day one day when heaven Day one day one noodle ss2
Day one day one noodle ss2 Day one day one noodles ss2
Day one day one noodles ss2 Chemistry unit 9 lesson 4
Chemistry unit 9 lesson 4 Ap chemistry thermochemistry frq
Ap chemistry thermochemistry frq Chemistry unit 7 molarity
Chemistry unit 7 molarity Unit 9 thermochemistry
Unit 9 thermochemistry Chemistry semester 2 review unit 12 thermochemistry
Chemistry semester 2 review unit 12 thermochemistry Molar concentration unit
Molar concentration unit