General Chem Review Quest Organic Chemistry Chemistry The


























- Slides: 48

General Chem Review Quest Organic Chemistry

Chemistry The study of matter and how it changes and interacts with other matter. Matter is anything that has mass and takes up space.

Properties of Matter Physical Properties: Density, melting point, boiling point, color, smell, etc. Chemical Properties: How the atoms are connected, for example characteristics in chemical reactions.

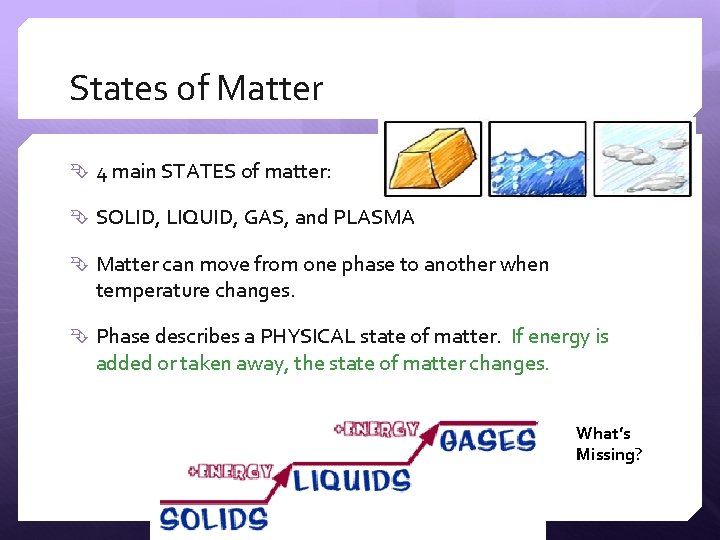
States of Matter 4 main STATES of matter: SOLID, LIQUID, GAS, and PLASMA Matter can move from one phase to another when temperature changes. Phase describes a PHYSICAL state of matter. If energy is added or taken away, the state of matter changes. What’s Missing?

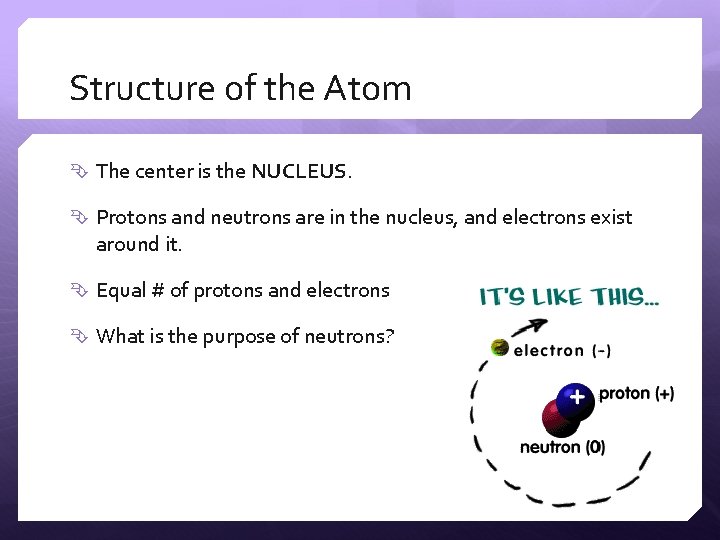
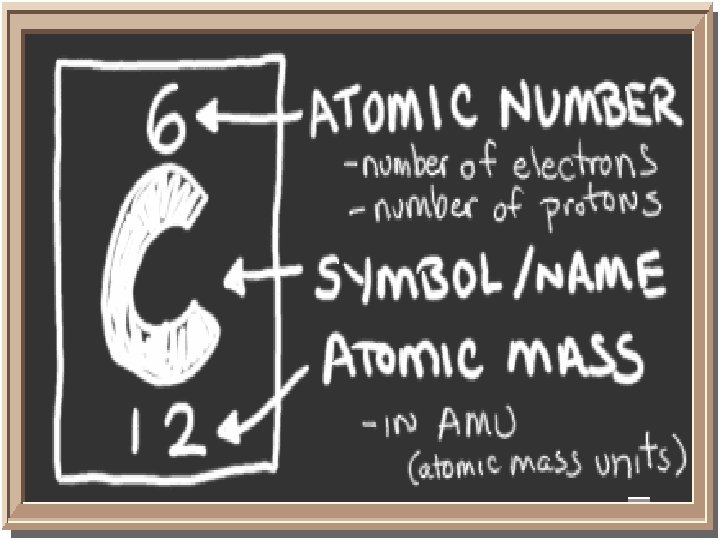
Atoms Smallest unit of matter Components are: PROTONS, NEUTRONS, and ELECTRONS Atoms are the basis for everything in the universe.

Structure of the Atom The center is the NUCLEUS. Protons and neutrons are in the nucleus, and electrons exist around it. Equal # of protons and electrons What is the purpose of neutrons?

Atoms are 3 D – maybe 4 D Atomic clocks tick slower near gravity Based on oscillations of the cesium atom Atomic clocks or orbital clocks are changing.

Time / Speed of Light/ Atoms Contrary to popular teaching, the speed of light appears to be slowing down. https: //www. youtube. com/watch? v =Mgp. I 3 FOHbvo Red shift and the atoms of the universe.

Atomic clouds Where are the electrons at any given moment? Where is my cat locked in my house?

Where does this energy for electrons come from? Electrons should run out of energy and slow down, but they don’t. This is due to zero point energy (ZPE) https: //www. youtube. com/watch? v=m 1 FUra. Ln 4 v 0

God’s input into the universe Colossians 1: 17 (NIV) He is before all things, and in him all things hold together.

Electrons contain energy and are grouped into orbitals according to how much energy they have. Similar energy levels get the same number. For example, 2 s and 2 p are grouped together. They have similar amounts of energy.

Electrons Similar energy levels are called SHELLS because the surround the nucleus like rings of an onion. Not all shells hold the same number or electrons.

Identify the use of this formula 2 2 n

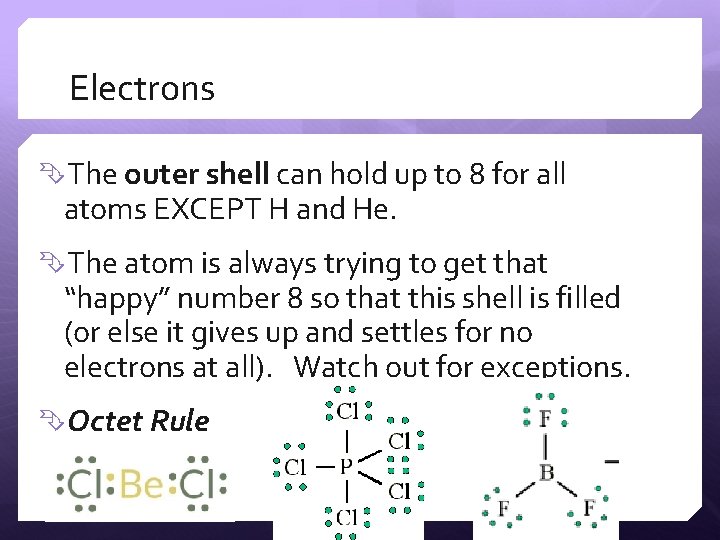
Electrons The outer shell can hold up to 8 for all atoms EXCEPT H and He. The atom is always trying to get that “happy” number 8 so that this shell is filled (or else it gives up and settles for no electrons at all). Watch out for exceptions. Octet Rule

Electrons That shell is called the VALENCE shell. Compounds can only be stable when that shell holds 0 or 8 electrons. (Exceptions) This is how bonding occurs.

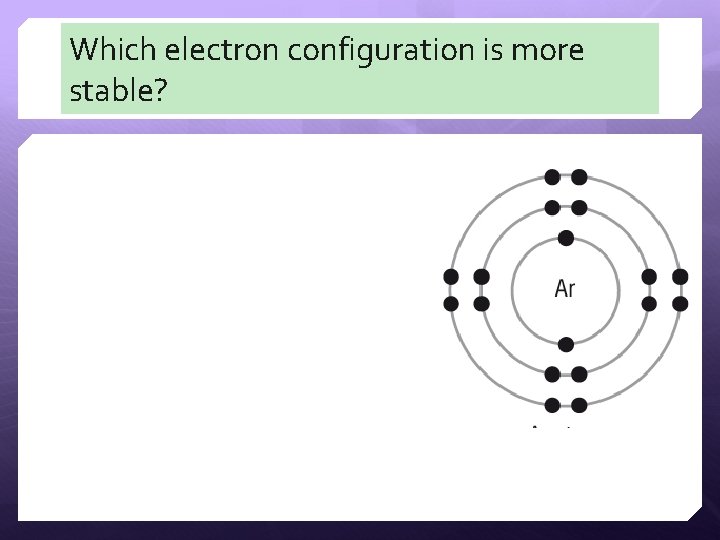
Which electron configuration is more stable?

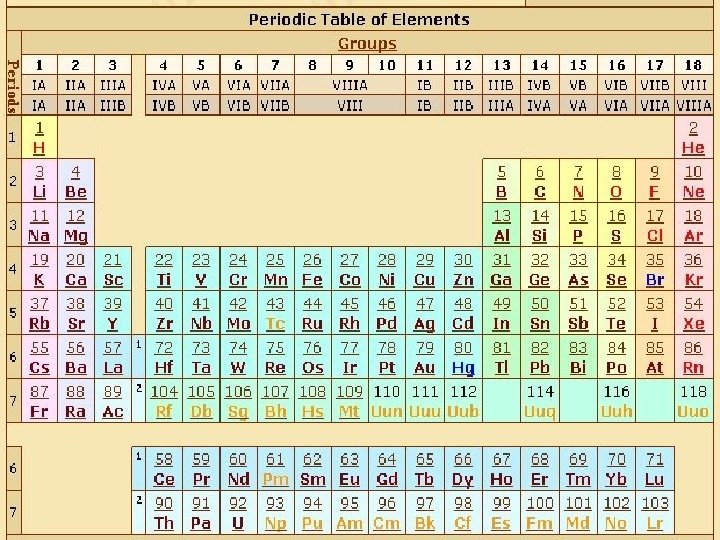
Periodic Table

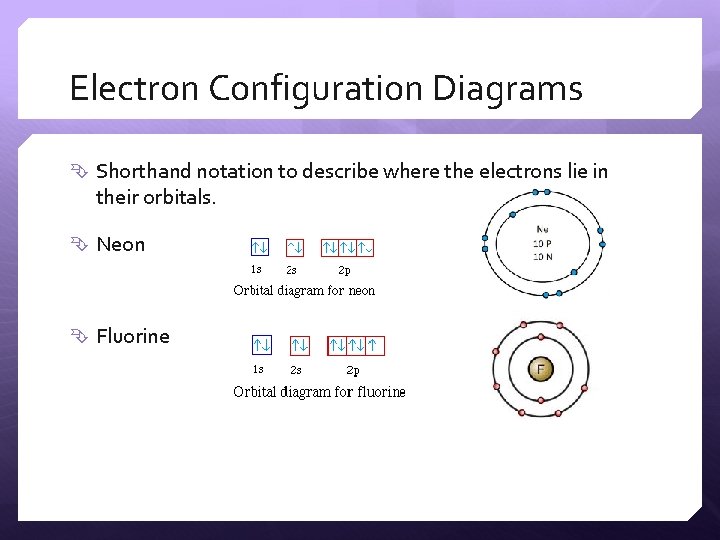
Electron Configuration Diagrams Shorthand notation to describe where the electrons lie in their orbitals. Neon Fluorine

Energy vs. Stability Since opposites attract, the electrons (negative) “want” to be closer to the nucleus where the protons (positive) reside. When they get closer to the nucleus, this is called a “lower” energy level because it has less energy. Less energy = more stable

Organization of the Periodic Table Periods: rows ACROSS the table. All the elements in a period share a common # of electron shells, or energy levels. Every element in the top row (the first period) has one shell for its electrons. All of the elements in the second row (the second period) have two shells for their electrons. It continues down the periodic table like that. At this time, the maximum number of shells is seven.

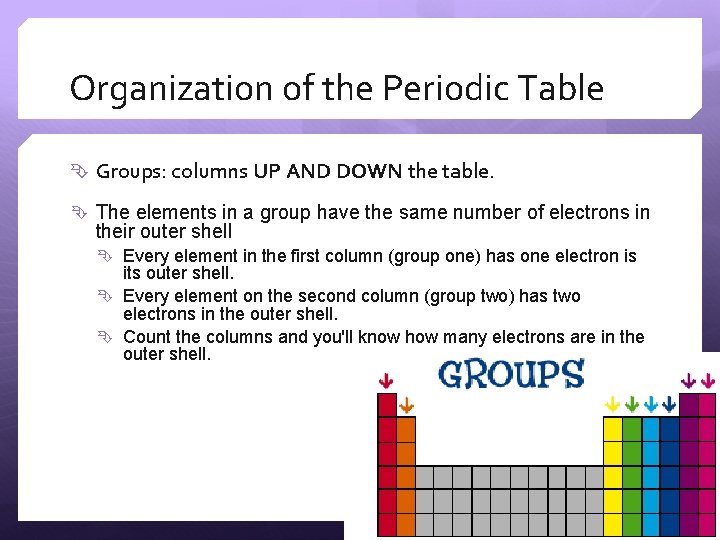
Organization of the Periodic Table Groups: columns UP AND DOWN the table. The elements in a group have the same number of electrons in their outer shell Every element in the first column (group one) has one electron is its outer shell. Every element on the second column (group two) has two electrons in the outer shell. Count the columns and you'll know how many electrons are in the outer shell.

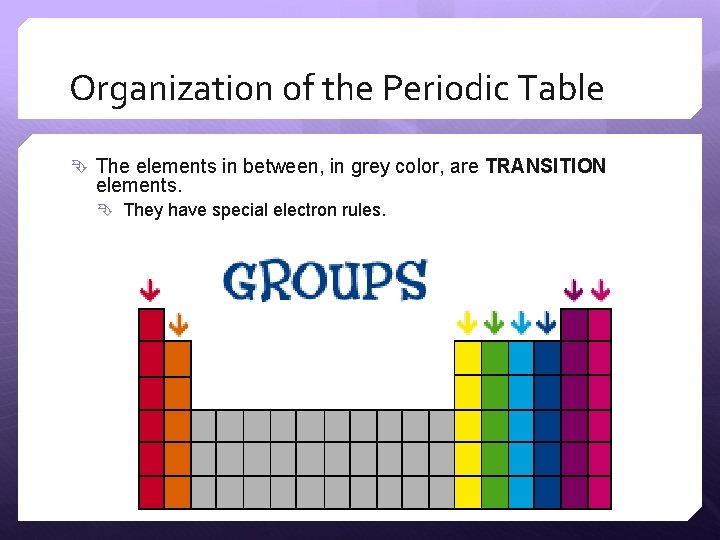
Organization of the Periodic Table The elements in between, in grey color, are TRANSITION elements. They have special electron rules.

Exceptions Hydrogen can have the talents and electrons of two groups, groups one and seven. Hydrogen is sometimes missing an electron, and sometimes it takes on an extra. Helium can only have two electrons in its outer shell. It is still grouped with noble gas elements that have eight.


Isotopes They are types of the same variety of atom. Remember the coke example

Meet Chemistry Cat He wanted to be an alchemist! Make up some isotopes of gold using the wooden model.

Let's use carbon (C) as an example. . . There a lot of carbon (C) atoms in the universe. The normal ones are called Carbon-12. Have 6 neutrons. Odd ones may have 7 or even 8 neutrons Carbon-14 Has 8 neutrons (2 extra). C-14 is considered to be an isotope of the element of Carbon (C). When C-14 loses the extra neutrons in radioactive DECAY, it will become C-12.

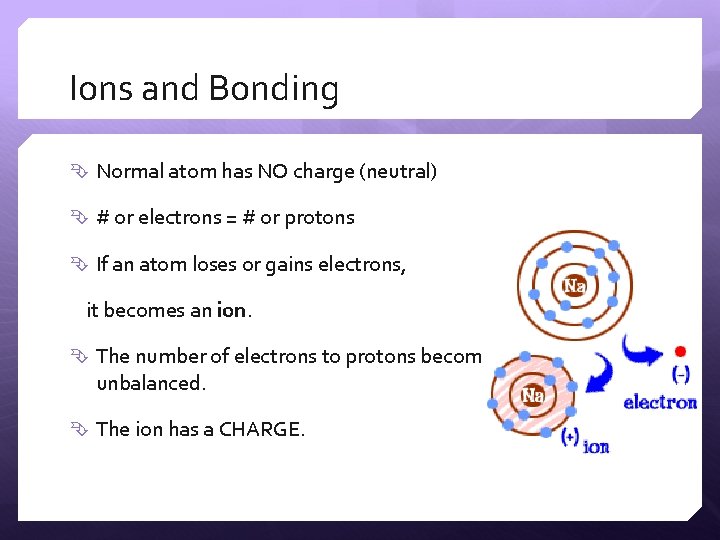
Ions and Bonding Normal atom has NO charge (neutral) # or electrons = # or protons If an atom loses or gains electrons, it becomes an ion. The number of electrons to protons becomes is is is unbalanced. The ion has a CHARGE.

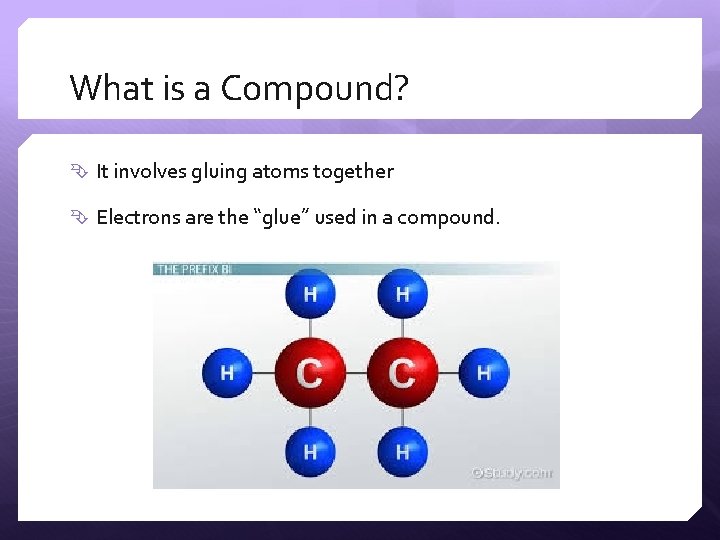
What is a Compound? It involves gluing atoms together Electrons are the “glue” used in a compound.

Build a Compound! One atom is bonded to another and another until a complete compound is formed. Use the model kit to build the following compounds: HCN – Hydrogen Cyanide NO – Nitrogen Monoxide NO 2 – Nitrogen Dioxide O 3 – Ozone CO 2 – Carbon Dioxide

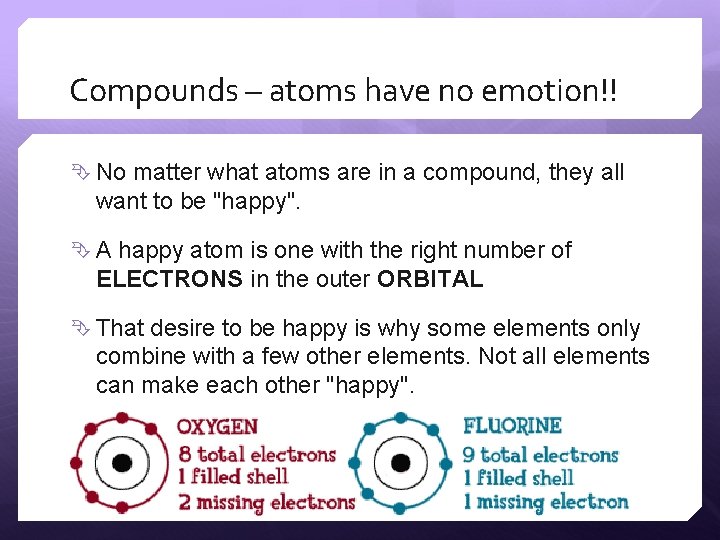
Compounds – atoms have no emotion!! No matter what atoms are in a compound, they all want to be "happy". A happy atom is one with the right number of ELECTRONS in the outer ORBITAL That desire to be happy is why some elements only combine with a few other elements. Not all elements can make each other "happy".

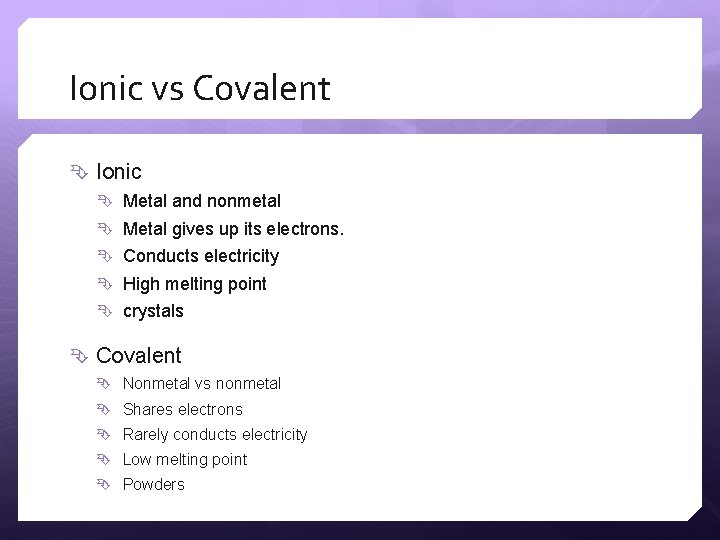
Ionic vs Covalent Ionic Metal and nonmetal Metal gives up its electrons. Conducts electricity High melting point crystals Covalent Nonmetal vs nonmetal Shares electrons Rarely conducts electricity Low melting point Powders

Covalent Compounds in O Chem Almost all compounds are covalent in organic chemistry. Therefore, it is important for you to have a solid understanding of how they behave.

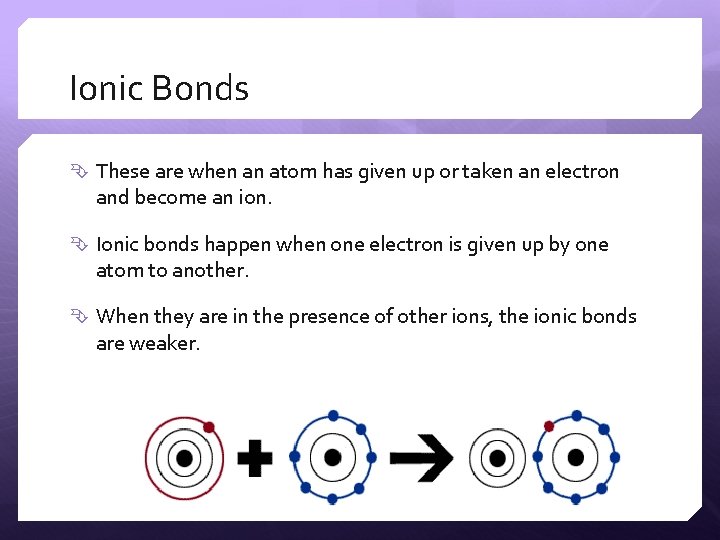
Ionic Bonds These are when an atom has given up or taken an electron and become an ion. Ionic bonds happen when one electron is given up by one atom to another. When they are in the presence of other ions, the ionic bonds are weaker.

Electrovalent Bonds ELECTROVALENCE is just another term for when an atom has become an ion. Electrovalent bonds mean the exact same thing as ionic bonds.

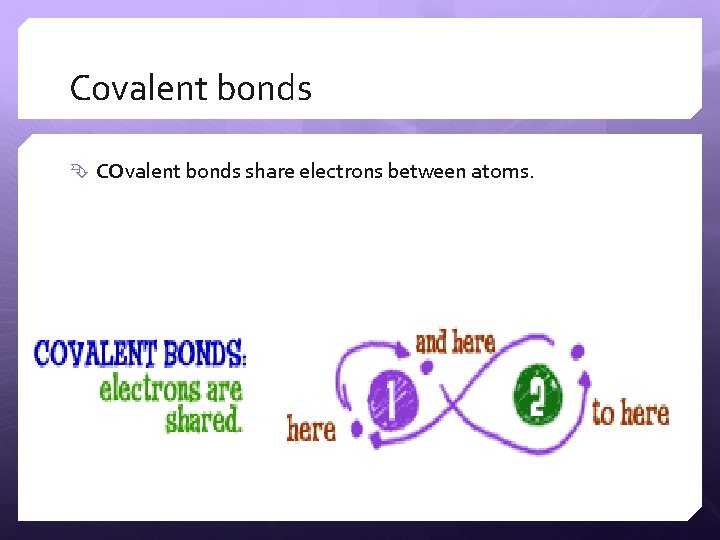
Covalent bonds COvalent bonds share electrons between atoms.

When sodium atoms bond with one oxygen atom there have to be two sodium atoms, each with an extra electron. Each of these Sodium atoms gives oxygen one electron, allowing oxygen to have a full shell with eight electrons. Is this an example of ionic or covalent bonding?

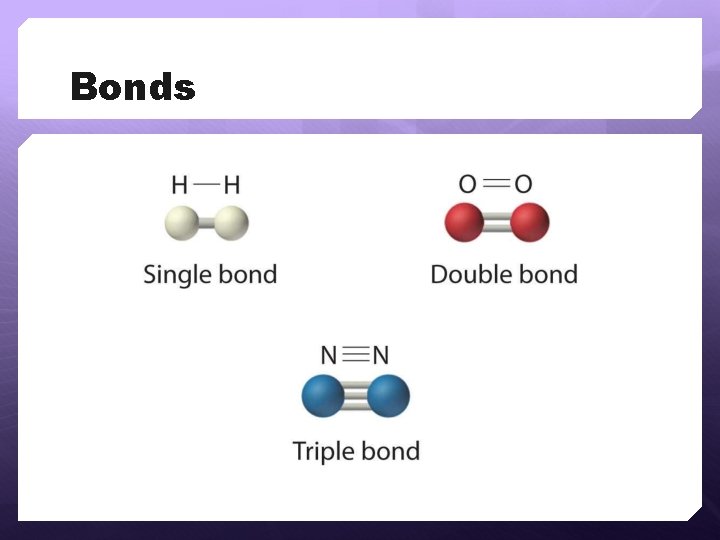
Bonds

How does bonding affect our world Flamibility HYDROGEN – bond is easy to break. flammable. Very OXYGEN – bonds harder to break, does not burn but supports combustion. NITROGEN – bonds much harder to break. flammable. Not

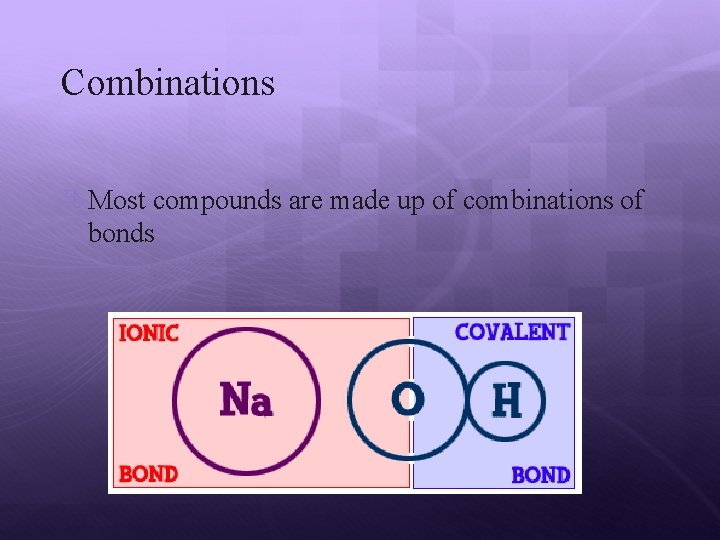
Combinations Most compounds are made up of combinations of bonds

You Tube video Ionic vs covalent compounds https: //www. youtube. com/watch? v=-u. Pgoq. Sl 8 vk

Reactions are: Electrons are the reason for chemical reactions! Reactions are simply bonds forming and bonds breaking. Electrons aligning between atoms forms a bond Removing electrons between two atoms breaks a bond.

Electrons for reactions n Where do electrons come from? n Electrons for reactions come from the atoms involved in the creation. n Electrons are the glue that sticks atoms together.

Reactions • In a reaction, a chemical change must occur. –Turn one compound into another. • It could be ions, molecules, or pure atoms • Single reactions often happen as part of a larger reactions. series of –Take something as simple as moving your arm. contraction of that muscle needs sugars Those sugars need metabolized… The for energy. • A lot has to happen just to perform a simple task!

Stoichiometry STOICHIOMETRY is the part of chemistry that studies amounts of substances that are involved in reactions What do you measure? Mass of Reactants (chemicals before the reaction) Mass of Products (chemicals after the reaction) Chemical Equations Molecular Weights of Reactants and Products Formulas of Various Compounds

Stoichiometry Example You start with two ions and end up with an ionic compound (Na. Cl). Na+ + Cl- --> Na. Cl It takes one atom of sodium (Na) to combine with one atom of chlorine (Cl) to make the salt. With stoichiometry, you can determine amounts of substances needed to fulfill the requirements of the reaction. Stoichiometry will tell you that if you have ten million atoms of sodium (Na) and only one atom of chlorine (Cl) you can only make one molecule of Sodium chloride (Na. Cl). 10, 000 Na+ + 1 Cl- --> Na. Cl + 9, 999 Na+ Nothing you can do will change that.

Labs As a class, please list the elements of a lab report and the key things it should contain. Inquiry Lab – all we give you is the compound. You must develop a process to determine if this is an organic or inorganic compound.
 Hept oct non dec
Hept oct non dec Induction chemistry
Induction chemistry Chapter 22 review organic chemistry section 1 answers
Chapter 22 review organic chemistry section 1 answers Ib chemistry organic chemistry
Ib chemistry organic chemistry Inorganic chemistry vs organic chemistry
Inorganic chemistry vs organic chemistry Gen chem review for ochem
Gen chem review for ochem Ap chemistry big idea 6 review answers
Ap chemistry big idea 6 review answers Ap chem electrochemistry review
Ap chem electrochemistry review Silkroad general quest
Silkroad general quest Cycloalkanes
Cycloalkanes Soap organic chemistry
Soap organic chemistry Ester organic chemistry
Ester organic chemistry Displayed formula
Displayed formula Rearranged most stable carbocation is
Rearranged most stable carbocation is Ee organic chemistry
Ee organic chemistry Ario organic chemistry
Ario organic chemistry Thermodynamic vs kinetic control
Thermodynamic vs kinetic control David klein organic chemistry 3rd edition
David klein organic chemistry 3rd edition Is alkane an organic compound
Is alkane an organic compound What is the leveling effect organic chemistry
What is the leveling effect organic chemistry Priority when naming organic compounds
Priority when naming organic compounds Organic chemistry lab report example
Organic chemistry lab report example Alkane organic chemistry
Alkane organic chemistry Organic chemistry grade 10
Organic chemistry grade 10 Organic chemistry
Organic chemistry Introduction to organic chemistry
Introduction to organic chemistry Kiliani fischer synthesis
Kiliani fischer synthesis How is cracking done
How is cracking done Meth eth prop
Meth eth prop Organic chemistry myanmar
Organic chemistry myanmar Propagation organic chemistry
Propagation organic chemistry High resolution
High resolution Hono organic chemistry
Hono organic chemistry Propyl bromide
Propyl bromide Organic chemistry topic 11
Organic chemistry topic 11 Organic chemistry reaction pathways
Organic chemistry reaction pathways Alkene alcohol naming
Alkene alcohol naming What is organic chemistry like
What is organic chemistry like Ch4o organic or inorganic
Ch4o organic or inorganic Organic chemistry vocabulary
Organic chemistry vocabulary Carboxylic acid h3o+ reaction
Carboxylic acid h3o+ reaction Extraction of caffeine from vivarin tablets lab report
Extraction of caffeine from vivarin tablets lab report A level chemistry ocr organic synthesis
A level chemistry ocr organic synthesis Saponifiable lipids example
Saponifiable lipids example Ario acidity
Ario acidity Percentage yield practice questions
Percentage yield practice questions Polarimetry organic chemistry
Polarimetry organic chemistry Organic chemistry third edition david klein
Organic chemistry third edition david klein Radicals
Radicals