02112020 Particles and Radiation Structure of the atom














- Slides: 65

02/11/2020 Particles and Radiation

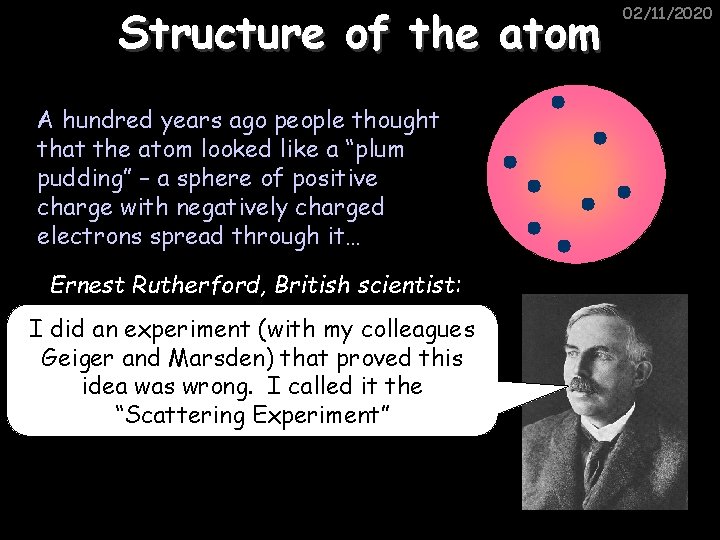
Structure of the atom A hundred years ago people thought that the atom looked like a “plum pudding” – a sphere of positive charge with negatively charged electrons spread through it… Ernest Rutherford, British scientist: I did an experiment (with my colleagues Geiger and Marsden) that proved this idea was wrong. I called it the “Scattering Experiment” 02/11/2020

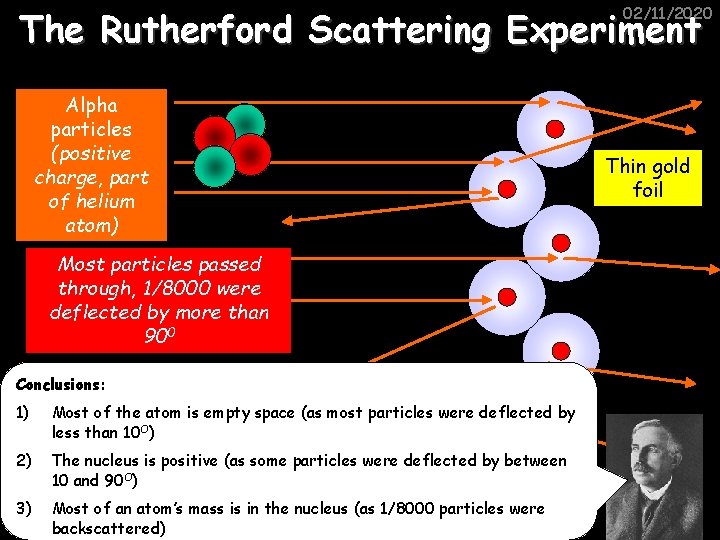
02/11/2020 The Rutherford Scattering Experiment Alpha particles (positive charge, part of helium atom) Most particles passed through, 1/8000 were deflected by more than 900 Conclusions: 1) Most of the atom is empty space (as most particles were deflected by less than 10 O) 2) The nucleus is positive (as some particles were deflected by between 10 and 90 O) 3) Most of an atom’s mass is in the nucleus (as 1/8000 particles were backscattered) Thin gold foil

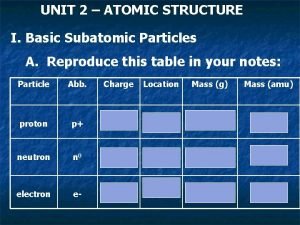
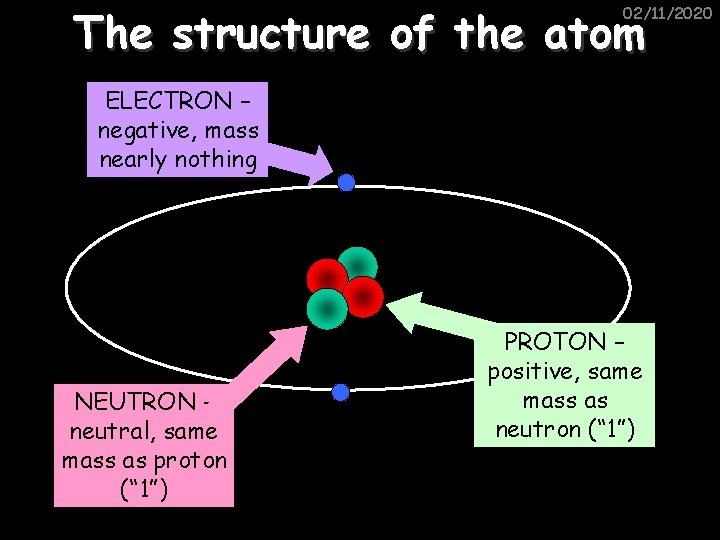
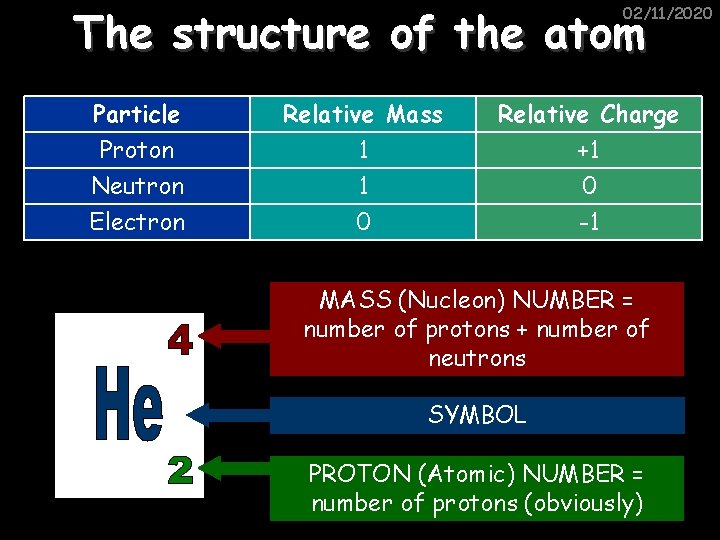
The structure of the atom 02/11/2020 ELECTRON – negative, mass nearly nothing NEUTRON – neutral, same mass as proton (“ 1”) PROTON – positive, same mass as neutron (“ 1”)

The structure of the atom 02/11/2020 Particle Proton Neutron Electron Relative Mass 1 1 0 Relative Charge +1 0 -1 MASS (Nucleon) NUMBER = number of protons + number of neutrons SYMBOL PROTON (Atomic) NUMBER = number of protons (obviously)

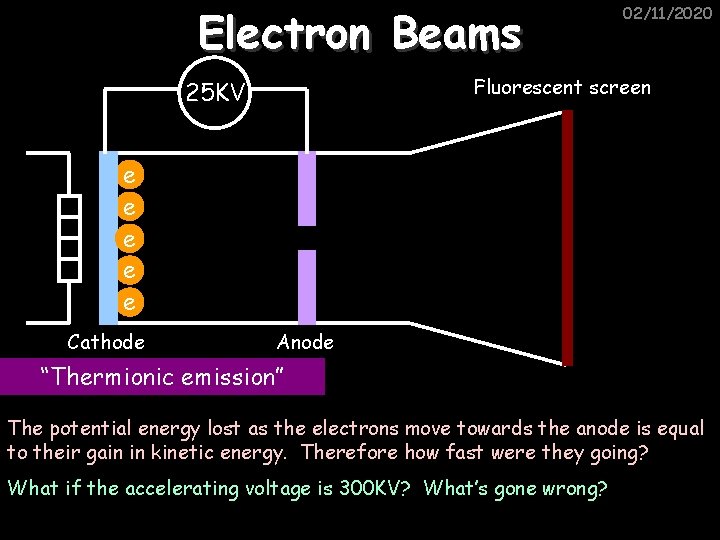
Electron Beams 02/11/2020 Fluorescent screen 25 KV e e e Cathode Anode “Thermionic emission” The potential energy lost as the electrons move towards the anode is equal to their gain in kinetic energy. Therefore how fast were they going? What if the accelerating voltage is 300 KV? What’s gone wrong?

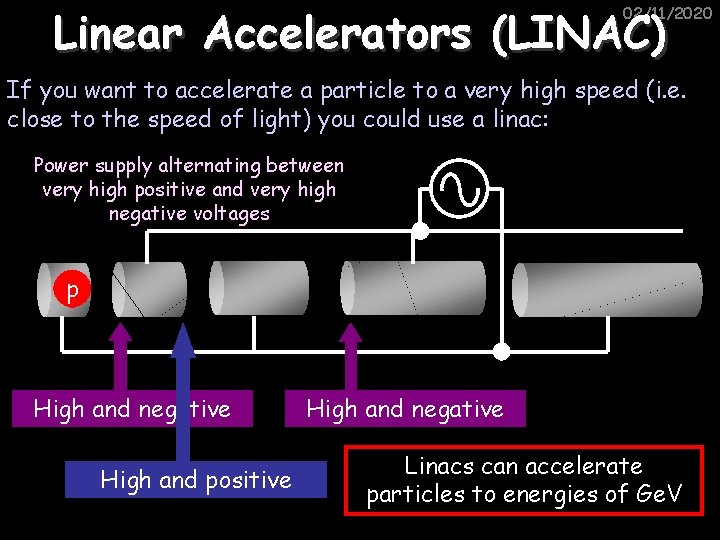
Linear Accelerators (LINAC) 02/11/2020 If you want to accelerate a particle to a very high speed (i. e. close to the speed of light) you could use a linac: Power supply alternating between very high positive and very high negative voltages p High and negative High and positive High and negative Linacs can accelerate particles to energies of Ge. V

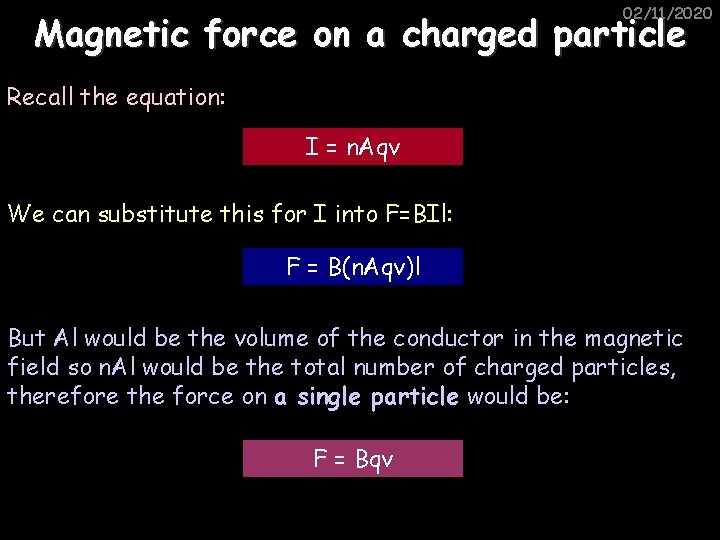
02/11/2020 Magnetic force on a charged particle Recall the equation: I = n. Aqv We can substitute this for I into F=BIl: F = B(n. Aqv)l But Al would be the volume of the conductor in the magnetic field so n. Al would be the total number of charged particles, therefore the force on a single particle would be: F = Bqv

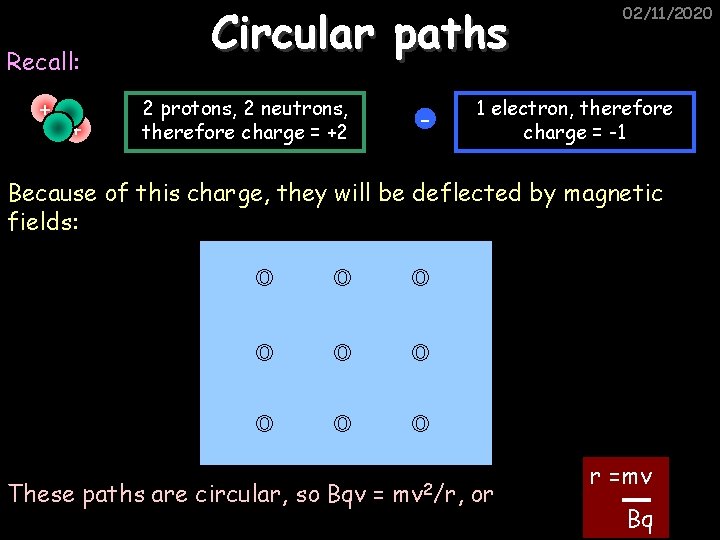
Recall: + + Circular paths 2 protons, 2 neutrons, therefore charge = +2 - 02/11/2020 1 electron, therefore charge = -1 Because of this charge, they will be deflected by magnetic fields: These paths are circular, so Bqv = mv 2/r, or r =mv Bq

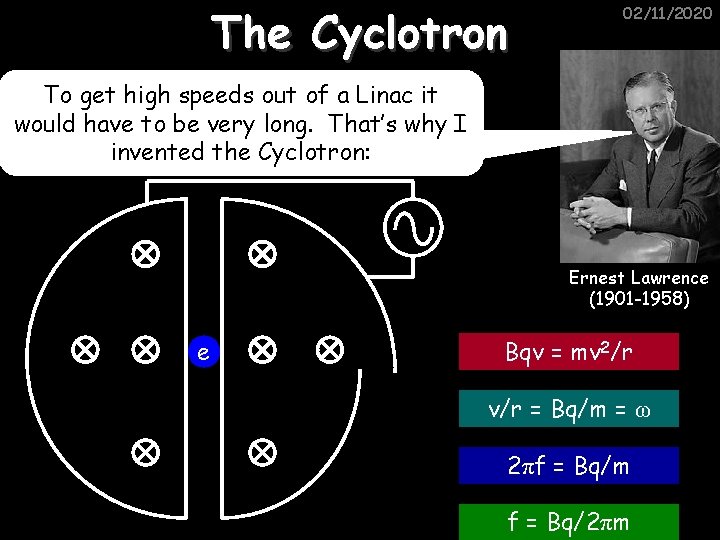
The Cyclotron 02/11/2020 To get high speeds out of a Linac it would have to be very long. That’s why I invented the Cyclotron: Ernest Lawrence (1901 -1958) e Bqv = mv 2/r v/r = Bq/m = ω 2πf = Bq/m f = Bq/2πm

Electric and Magnetic Fields in Accelerators and Detectors 02/11/2020 Key facts: • Electric fields are used to accelerate charged particles across the gap (in a LINAC) or between the dees (in a cyclotron). • Magnetic fields are used in cyclotrons only to provide a centripetal force and therefore make the charged particle move in a curved path. • Electric fields can be used in detectors to determine the charge on the particle by seeing which plate the particle is attracted to. • Magnetic fields can also be used to determine the charge on a particle using Fleming’s Left Hand Rule.

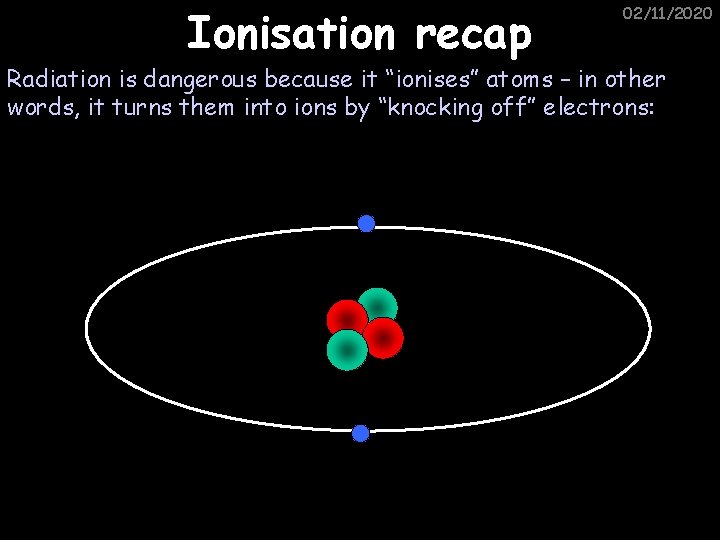
Ionisation recap 02/11/2020 Radiation is dangerous because it “ionises” atoms – in other words, it turns them into ions by “knocking off” electrons:

02/11/2020 Facts about accelerators and detectors Targets: A simple target could be liquid hydrogen (i. e. many protons). Beams: Having two beams collide at opposite directions is more useful – the particles have zero momentum before. However, this is harder to achieve in practice. Detectors: The charged particles these collisions produce will ionise a liquid or gas. • A bubble chamber can detect charged particles because bubbles form around the ions left behind. • A cloud chamber works by condensing alcohol on ions to make the particle path visible. • Spark and drift chambers work by detecting currents due to the ions and using a computer to reconstruct the particle paths.

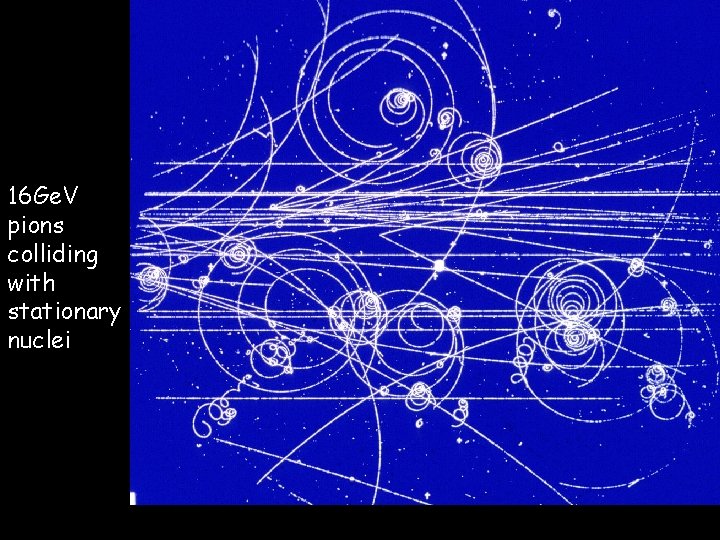
Smashing atoms 02/11/2020 The same principle applies to all matter – fast beams of particles like electrons can be used to find out what other particles are made of. The CERN lab in Geneva (27 km circumference). Some images…

02/11/2020

02/11/2020

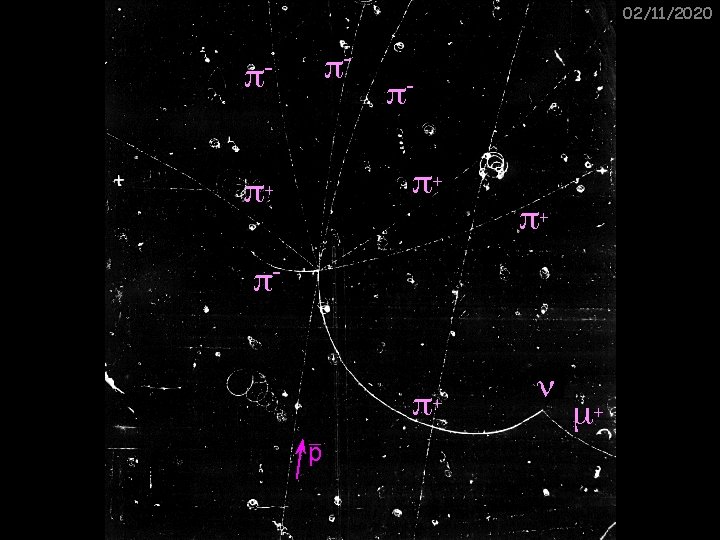
02/11/2020 16 Ge. V pions colliding with stationary nuclei

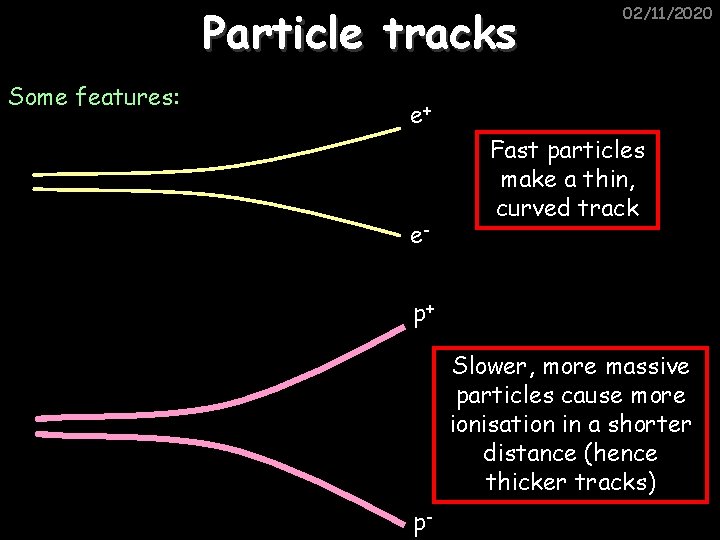
Particle tracks Some features: 02/11/2020 e+ e- Fast particles make a thin, curved track p+ Slower, more massive particles cause more ionisation in a shorter distance (hence thicker tracks) p-

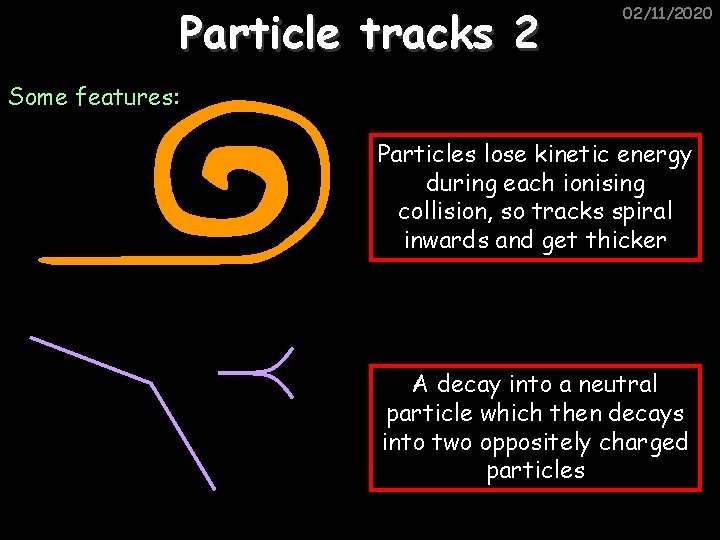
Particle tracks 2 02/11/2020 Some features: Particles lose kinetic energy during each ionising collision, so tracks spiral inwards and get thicker A decay into a neutral particle which then decays into two oppositely charged particles

Energy-mass equivalence 02/11/2020 Energy has a mass (and vice versa). We can calculate how much energy a given mass is worth using my famous formula: ∆E=∆mc 2 Einstein (1879 -1955) 1) Alex has a mass of 85 kg. How much energy is this? 2) A 0. 5 kg bowl of water is heated and gains about 10 KJ of energy. How much “mass” has it gained? 3) A Big Mac has a mass of 215 g. The average power usage of the UK is 40 GW. How long could a Big Mac power the UK for if it could be turned into energy?

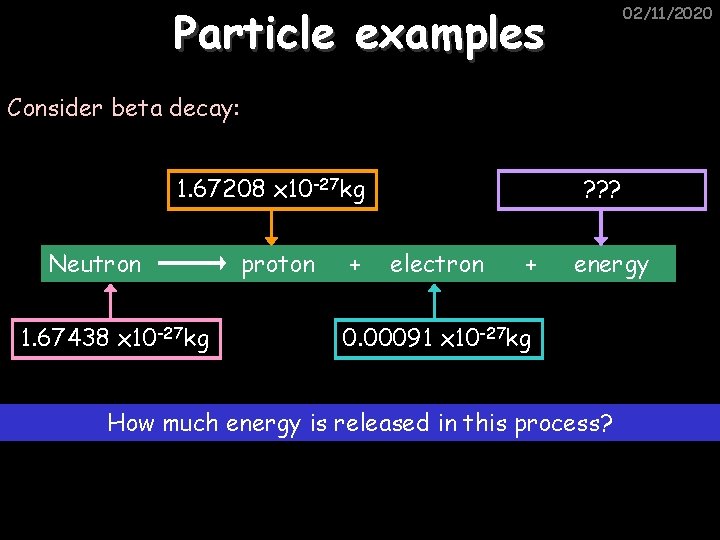
Particle examples 02/11/2020 Consider beta decay: 1. 67208 x 10 -27 kg Neutron 1. 67438 x 10 -27 kg proton + ? ? ? electron + energy 0. 00091 x 10 -27 kg How much energy is released in this process?

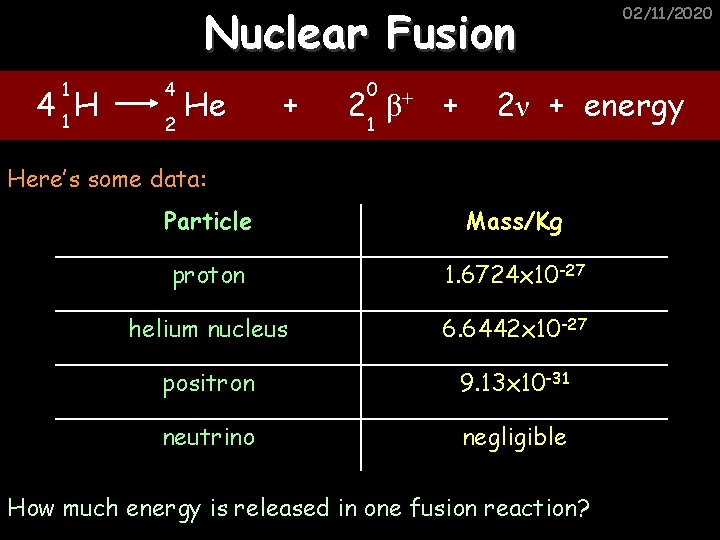
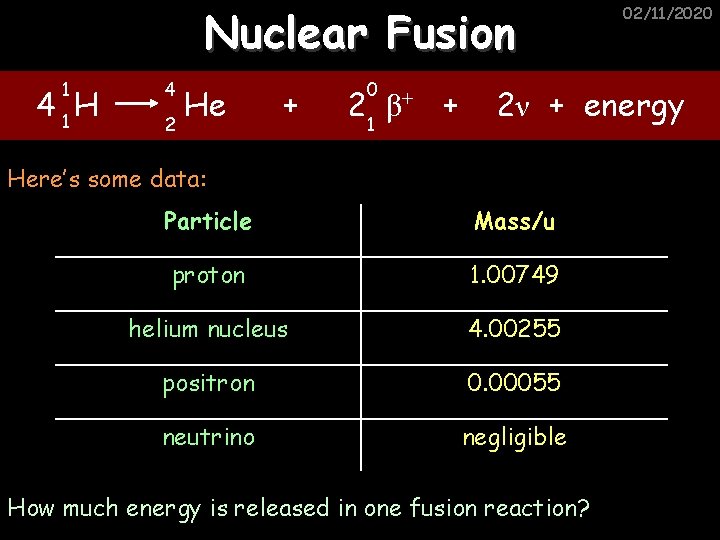
Nuclear Fusion 1 4 1 H 4 He 2 + 0 2 1 β+ + 02/11/2020 2ν + energy Here’s some data: Particle Mass/Kg proton 1. 6724 x 10 -27 helium nucleus 6. 6442 x 10 -27 positron 9. 13 x 10 -31 neutrino negligible How much energy is released in one fusion reaction?

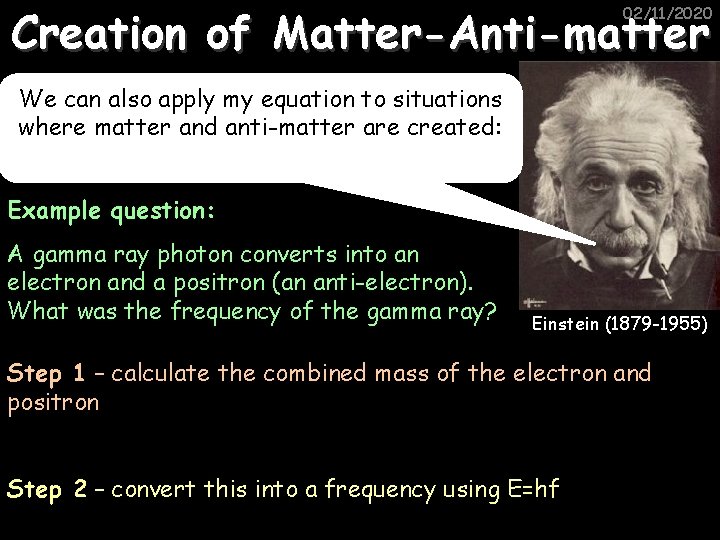
Creation of Matter-Anti-matter 02/11/2020 We can also apply my equation to situations where matter and anti-matter are created: Example question: A gamma ray photon converts into an electron and a positron (an anti-electron). What was the frequency of the gamma ray? Einstein (1879 -1955) Step 1 – calculate the combined mass of the electron and positron Step 2 – convert this into a frequency using E=hf

02/11/2020 The Unified Atomic Mass Unit (u) 1 u = 1/12 th the mass of carbon = 1. 66 x 10 -27 kg According to a (good) periodic table, the relative atomic mass of hydrogen is 1. 0079. This really means 1. 0079 u, or 1. 67 x 10 -27 kg. 1) What is the mass of sodium if its relative atomic mass is 22. 99? 3. 82 x 10 -26 kg 2) What is 1 u in joules? 3) What is 1 u in e. V? 1. 49 x 10 -10 J 934 Me. V

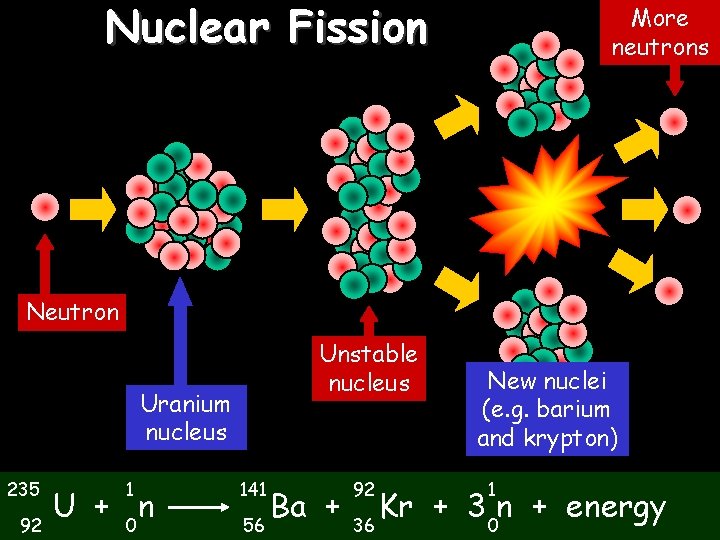
Nuclear Fission 02/11/2020 More neutrons Neutron Unstable nucleus Uranium nucleus 235 92 1 U + 0 n 141 56 Ba + 92 36 New nuclei (e. g. barium and krypton) 1 Kr + 3 0 n + energy

Nuclear Fusion 1 4 1 H 4 He 2 + 0 2 1 β+ + 02/11/2020 2ν + energy Here’s some data: Particle Mass/u proton 1. 00749 helium nucleus 4. 00255 positron 0. 00055 neutrino negligible How much energy is released in one fusion reaction?

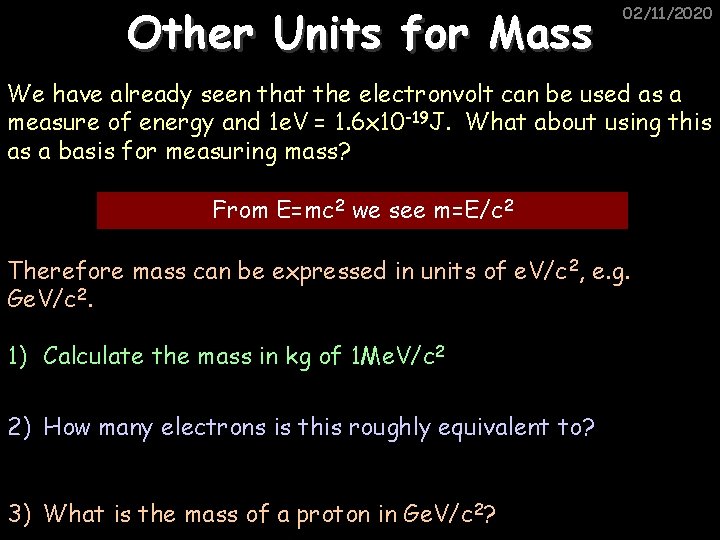
Other Units for Mass 02/11/2020 We have already seen that the electronvolt can be used as a measure of energy and 1 e. V = 1. 6 x 10 -19 J. What about using this as a basis for measuring mass? From E=mc 2 we see m=E/c 2 Therefore mass can be expressed in units of e. V/c 2, e. g. Ge. V/c 2. 1) Calculate the mass in kg of 1 Me. V/c 2 2) How many electrons is this roughly equivalent to? 3) What is the mass of a proton in Ge. V/c 2?

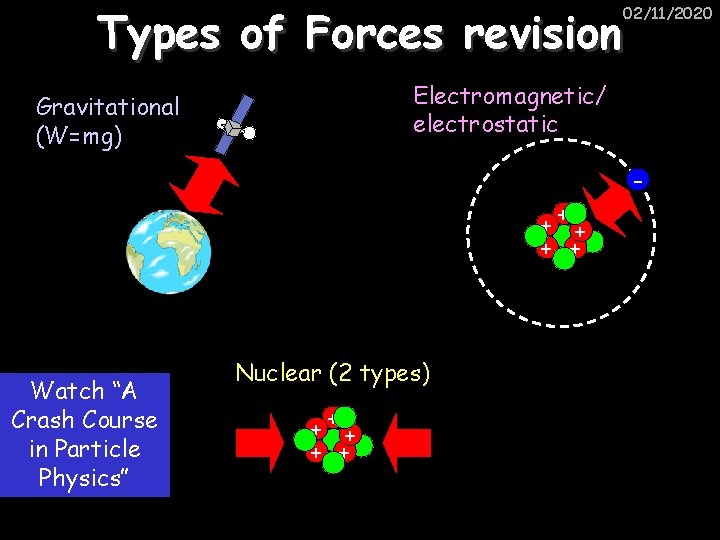
Types of Forces revision 02/11/2020 Electromagnetic/ electrostatic Gravitational (W=mg) +++ + + Watch “A Crash Course in Particle Physics” Nuclear (2 types) +++ + +

Fundamental particles 02/11/2020 People used to think that the atom was the smallest thing in the universe but my alpha particle scattering experiment showed that the atom is made of smaller things called protons, neutrons and electrons. Ah yes, but are protons, electrons and neutrons made up of even smaller particles? We need to investigate this using electrons with seriously high energies. Richard Feynman, 1918 -1988

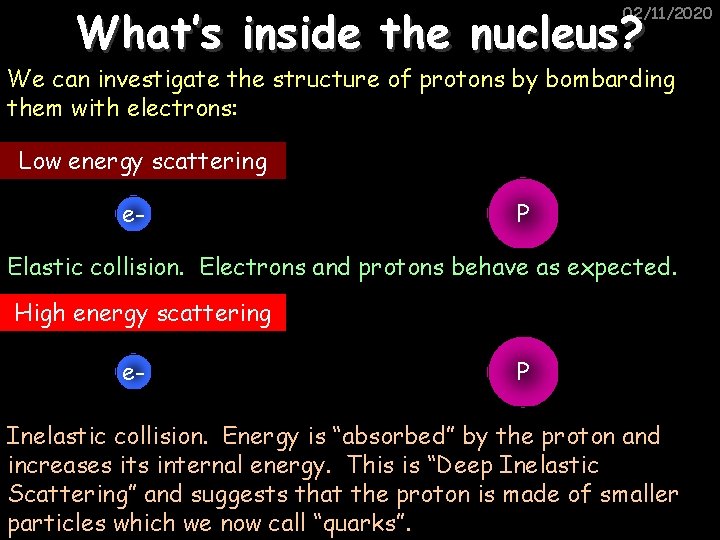
What’s inside the nucleus? 02/11/2020 We can investigate the structure of protons by bombarding them with electrons: Low energy scattering e- P Elastic collision. Electrons and protons behave as expected. High energy scattering e- P Inelastic collision. Energy is “absorbed” by the proton and increases its internal energy. This is “Deep Inelastic Scattering” and suggests that the proton is made of smaller particles which we now call “quarks”.

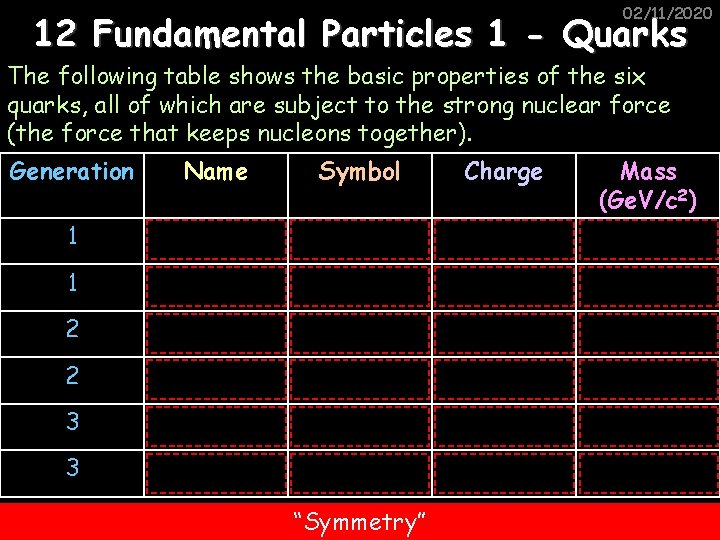
02/11/2020 12 Fundamental Particles 1 - Quarks The following table shows the basic properties of the six quarks, all of which are subject to the strong nuclear force (the force that keeps nucleons together). Generation Name Symbol Charge 1 Up u +2/3 Mass (Ge. V/c 2) 0. 003 1 Down d -1/3 0. 006 2 Strange s -1/3 0. 1 2 Charm c +2/3 1. 3 3 Bottom b -1/3 4. 3 3 Top t +2/3 175 “Symmetry”

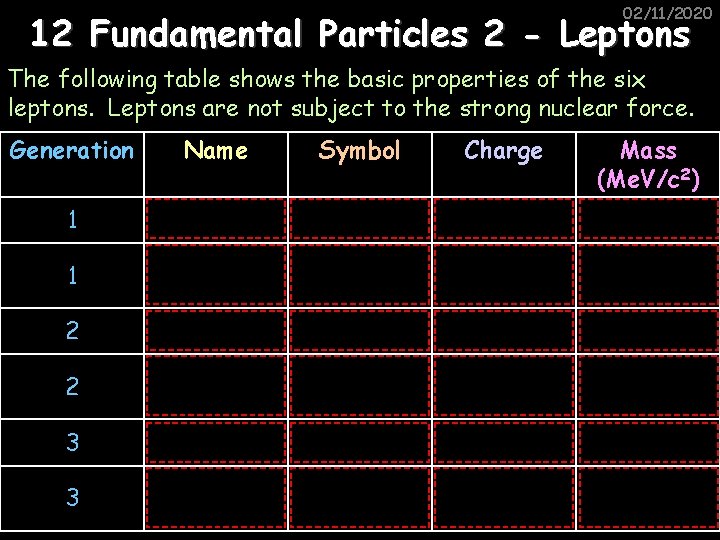
02/11/2020 12 Fundamental Particles 2 - Leptons The following table shows the basic properties of the six leptons. Leptons are not subject to the strong nuclear force. Generation Name Symbol Charge Mass (Me. V/c 2) 1 Electron e- -1 0. 511 1 Electron neutrino νe 0 0 (‹ 2 x 10 -6) 2 Muon μ -1 106 2 Muon neutrino νμ 0 0 (‹ 0. 17) 3 Tau τ -1 1780 3 Tau neutrino ντ 0 0 (‹ 20)

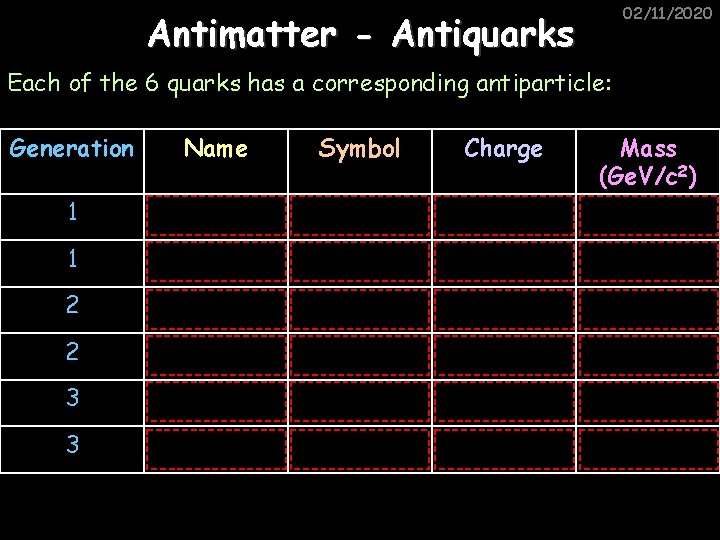
02/11/2020 Antimatter - Antiquarks Each of the 6 quarks has a corresponding antiparticle: Generation Name Symbol Charge 1 Anti-up u -2/3 Mass (Ge. V/c 2) 0. 003 1 Anti-down d +1/3 0. 006 2 Anti-strange s +1/3 0. 1 2 Anti-charm c -2/3 1. 3 3 Anti-bottom b +1/3 4. 3 3 Anti-top t -2/3 175

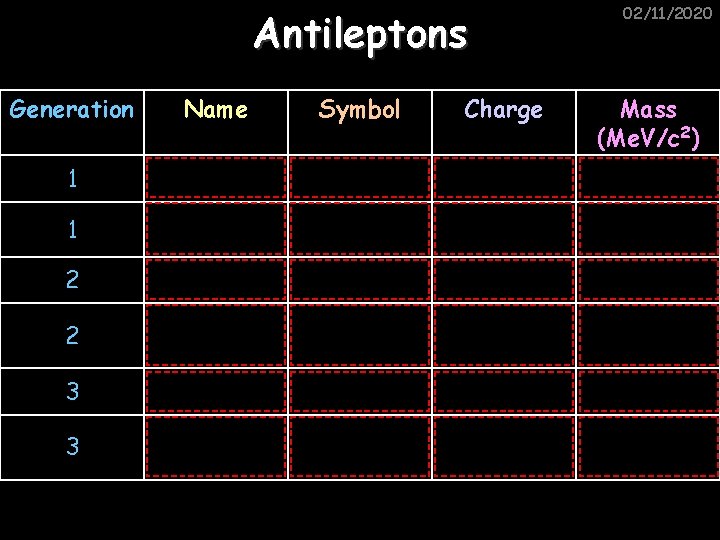
02/11/2020 Antileptons Generation Name Symbol Charge Mass (Me. V/c 2) 1 Positron e+ +1 0. 511 1 Anti-electron neutrino νe 0 0 (‹ 2 x 10 -6) 2 Anti-muon μ +1 106 2 Anti-muon neutrino νμ 0 0 (‹ 0. 17) 3 Anti-tau τ +1 1780 3 Anti-tau neutrino ντ 0 0 (‹ 20)

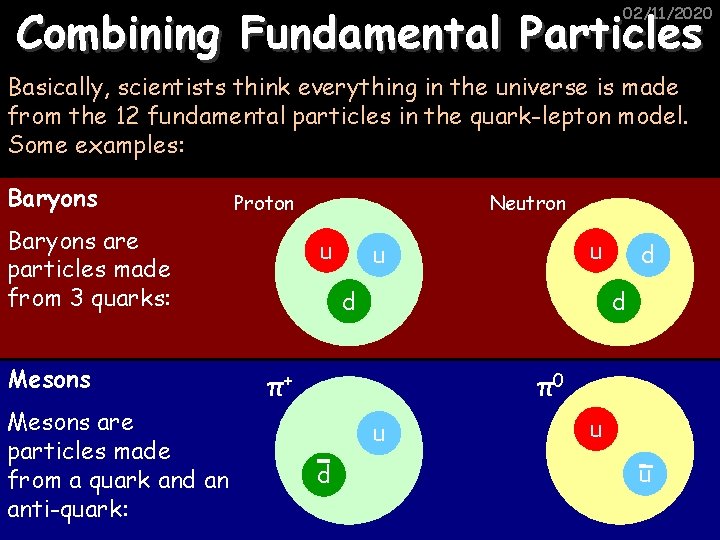
Combining Fundamental Particles 02/11/2020 Basically, scientists think everything in the universe is made from the 12 fundamental particles in the quark-lepton model. Some examples: Baryons Proton Baryons are particles made from 3 quarks: Mesons are particles made from a quark and an anti-quark: Neutron u u u d d d π+ π0 u d u u

Hadrons 02/11/2020 Definition – a hadron is a particle that feels the strong force. In other words, baryons and mesons are also called hadrons because they are made of quarks and therefore feel the strong force.

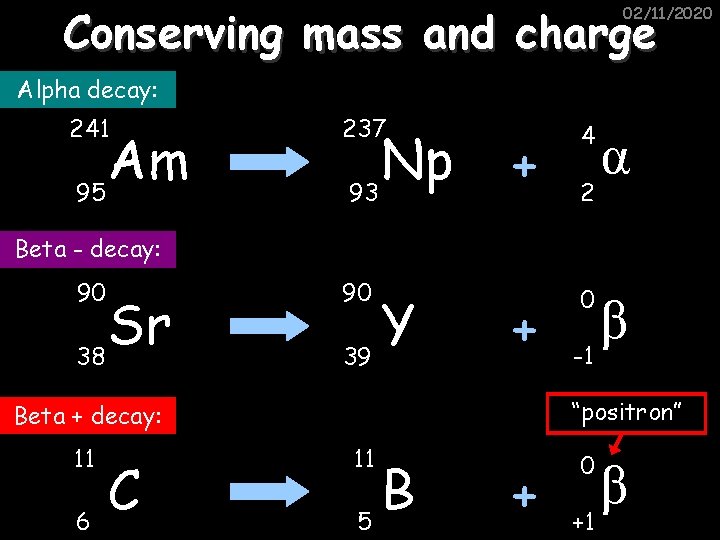
Conserving mass and charge 02/11/2020 Alpha decay: 241 Am 95 237 Np 93 + 4 + 0 2 α Beta - decay: 90 Sr 38 90 Y 39 “positron” Beta + decay: 11 6 C β -1 11 B 5 + 0 +1 β

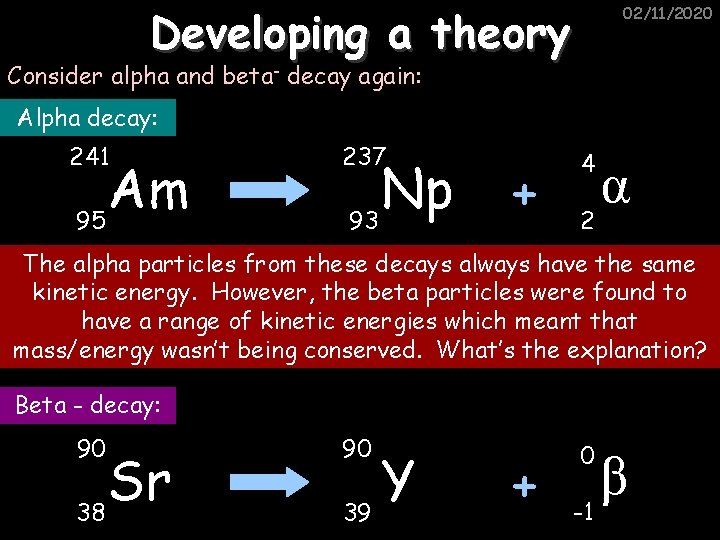
Developing a theory 02/11/2020 Consider alpha and beta- decay again: Alpha decay: 241 Am 95 237 Np 93 + 4 2 α The alpha particles from these decays always have the same kinetic energy. However, the beta particles were found to have a range of kinetic energies which meant that mass/energy wasn’t being conserved. What’s the explanation? Beta - decay: 90 Sr 38 90 Y 39 + 0 β -1

The Answer… 02/11/2020 There must be some other particles being produced as well, hence the neutrino: 90 90 0 0 Sr 38 β ν Y + + e 0 -1 39 The energy of the neutrino will vary so that mass/energy is conserved. Q. What are the mass and charge numbers for the neutrino?

Conserving all Properties 02/11/2020 In every reaction or decay the following things all need to be conserved: 1) Mass/energy 2) Charge 3) Momentum 4) Lepton number 5) Baryon number 6) Strangeness

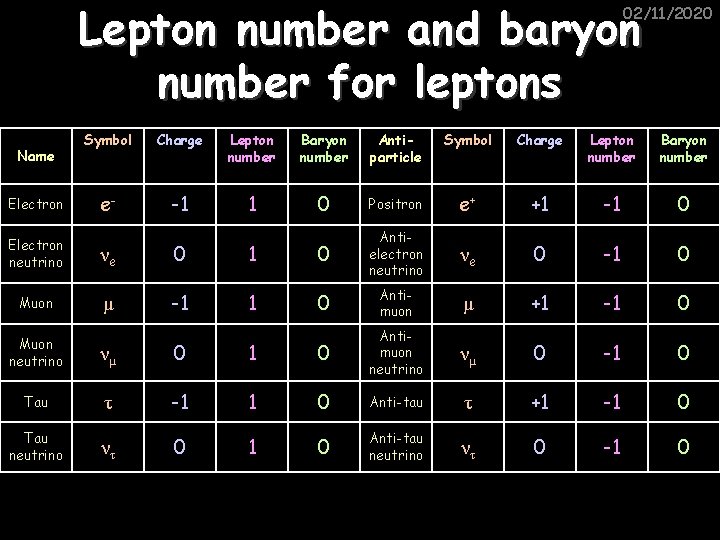
Lepton number and baryon number for leptons 02/11/2020 Name Electron Symbol Charge Lepton number Baryon number Antiparticle Symbol Charge Lepton number Baryon number e- -1 1 0 Positron e+ +1 -1 0 νe 0 -1 0 Electron neutrino νe 0 1 0 Antielectron neutrino Muon μ -1 1 0 Antimuon μ +1 -1 0 νμ 0 -1 0 Muon neutrino νμ 0 1 0 Antimuon neutrino Tau τ -1 1 0 Anti-tau τ +1 -1 0 Tau neutrino ντ 0 1 0 Anti-tau neutrino ντ 0 -1 0

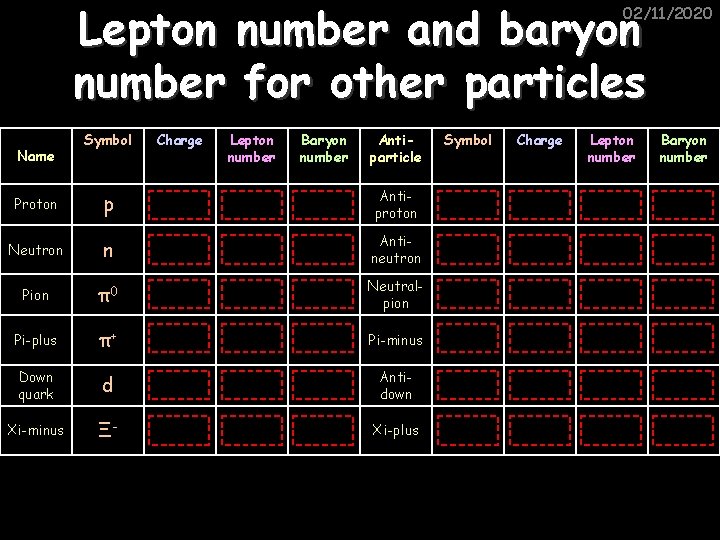
Lepton number and baryon number for other particles 02/11/2020 Symbol Charge Lepton number Baryon number Antiparticle Symbol Charge Lepton number Baryon number Proton p +1 0 +1 Antiproton p -1 0 -1 Neutron n 0 0 +1 Antineutron n 0 0 -1 Pion π0 0 Neutralpion π0 0 Pi-plus π+ +1 0 0 Pi-minus π- -1 0 0 Down quark d -1/3 0 +1/3 Antidown d +1/3 0 -1/3 Xi-minus Ξ- -1 0 +1 Xi-plus Ξ+ +1 0 -1 Name

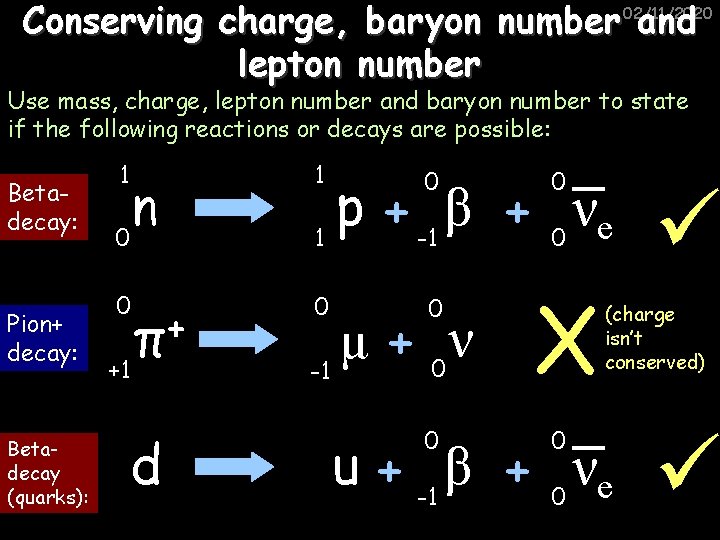
Conserving charge, baryon number and lepton number 02/11/2020 Use mass, charge, lepton number and baryon number to state if the following reactions or decays are possible: Betadecay: Pion+ decay: Betadecay (quarks): 1 n 0 1 0 p β ν + + 1 -1 0 e 0 0 0 +1 + π d ν μ + 0 -1 0 0 X 0 (charge isn’t conserved) u + -1 β + 0 νe

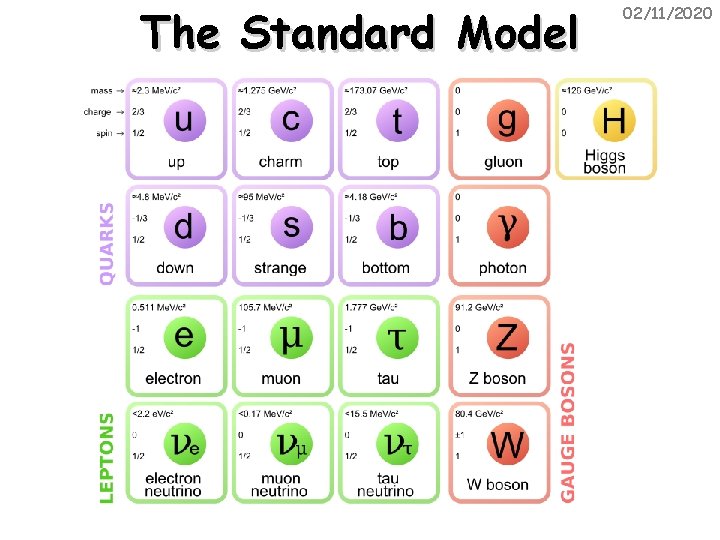
The Standard Model 02/11/2020

02/11/2020 Particle Theory and Wave Theory Light can be reflected, refracted and diffracted. These three things are called “wave behaviour” so light must travel as a wave. It makes sense! Isaac Newton, 1643 -1727 Ah yes, but light and other EM radiations also demonstrate some “particle behaviour” so many scientists say that it travels as a set of particles called photons. Here’s the evidence… Max Planck, 1858 -1947

02/11/2020 Introduction to Wave Particle Duality Some basic principles: 1) The wavelength of blue light is around 400 nm (4 x 10 -7 m) 2) The wavelength of red light is around 650 nm (6. 5 x 10 -7 m) 3) Therefore blue light is higher frequency than red light 4) Light is treated as being a wave. Therefore the amount of energy a light wave contains should depend on its intensity or brightness.

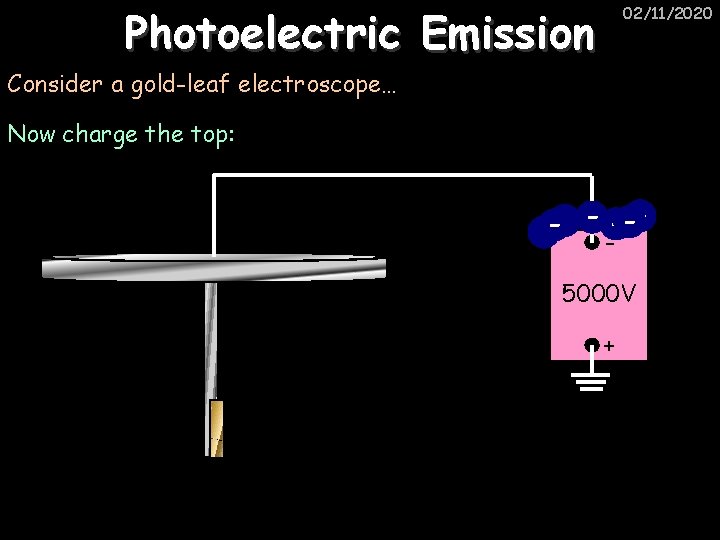
Photoelectric Emission 02/11/2020 Consider a gold-leaf electroscope… Now charge the top: 5000 V +

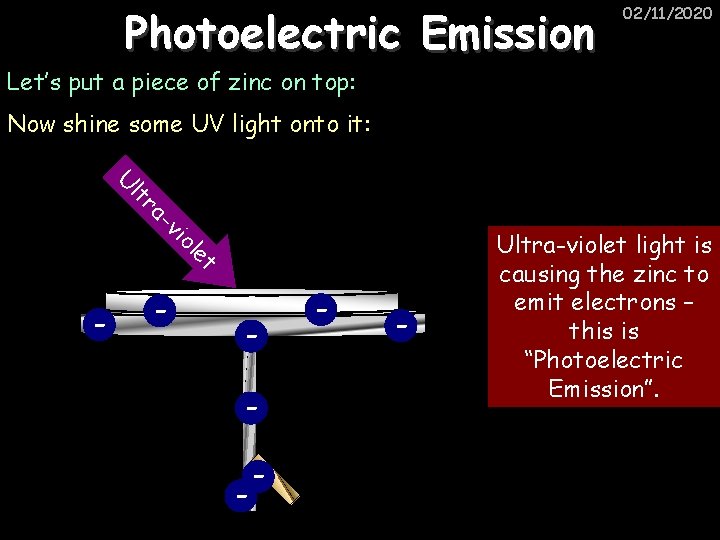
Photoelectric Emission 02/11/2020 Let’s put a piece of zinc on top: Now shine some UV light onto it: lt U et ol vi ra - - - Ultra-violet light is causing the zinc to emit electrons – this is “Photoelectric Emission”.

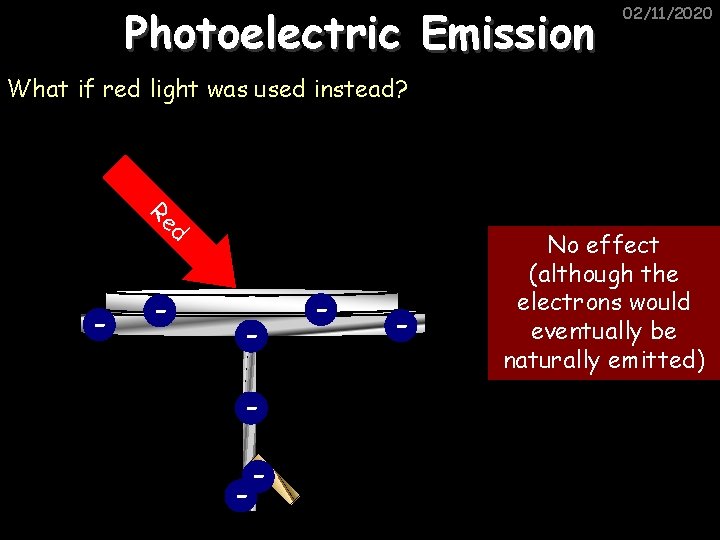
Photoelectric Emission 02/11/2020 What if red light was used instead? Re d - - - No effect (although the electrons would eventually be naturally emitted)

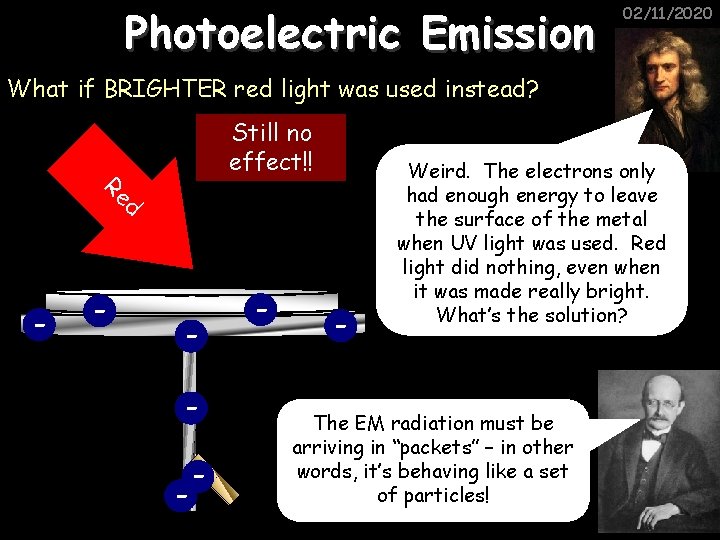
Photoelectric Emission 02/11/2020 What if BRIGHTER red light was used instead? Still no effect!! Re d - - - Weird. The electrons only had enough energy to leave the surface of the metal when UV light was used. Red light did nothing, even when it was made really bright. What’s the solution? The EM radiation must be arriving in “packets” – in other words, it’s behaving like a set of particles!

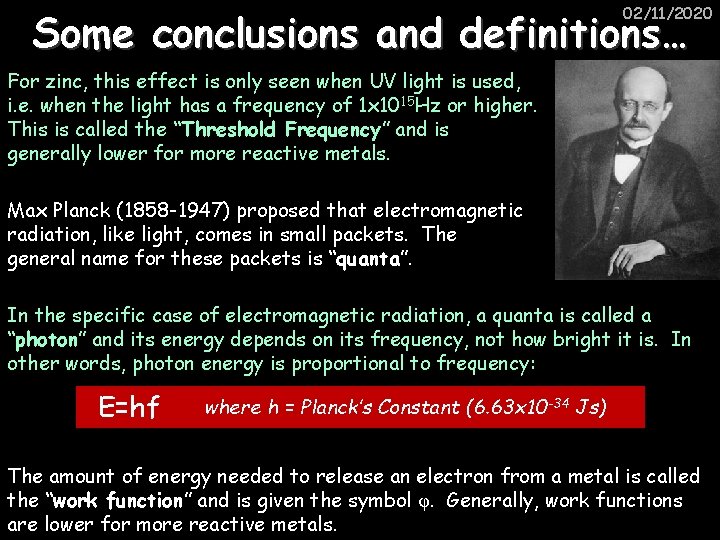
02/11/2020 Some conclusions and definitions… For zinc, this effect is only seen when UV light is used, i. e. when the light has a frequency of 1 x 1015 Hz or higher. This is called the “Threshold Frequency” and is generally lower for more reactive metals. Max Planck (1858 -1947) proposed that electromagnetic radiation, like light, comes in small packets. The general name for these packets is “quanta”. In the specific case of electromagnetic radiation, a quanta is called a “photon” and its energy depends on its frequency, not how bright it is. In other words, photon energy is proportional to frequency: E=hf where h = Planck’s Constant (6. 63 x 10 -34 Js) The amount of energy needed to release an electron from a metal is called the “work function” and is given the symbol φ. Generally, work functions are lower for more reactive metals.

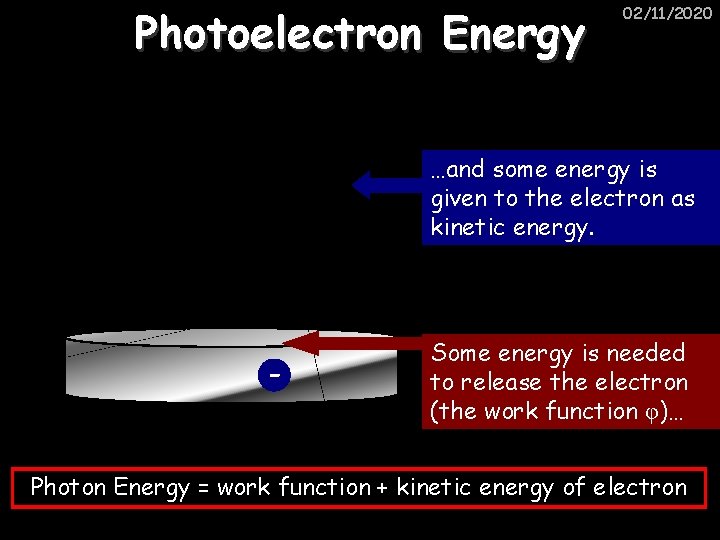
Photoelectron Energy 02/11/2020 …and some energy is given to the electron as kinetic energy. - Some energy is needed to release the electron (the work function φ)… Photon Energy = work function + kinetic energy of electron

Calculating Photon Energy 02/11/2020 Remember that the energy of a photon is proportional to its frequency, so E=hf, where h = Planck’s Constant = 6. 63 x 10 -34 Js. On the previous slide we said that… Photon energy = work function + kinetic energy of electron Therefore this equation becomes: hf = φ + 1/2 mv 2

The Electronvolt 02/11/2020 Recall the equation W=QV… This equation states that the work done on an electron (of charge 1. 6 x 10 -19 C) as it moves through a potential difference of 1 V is given by: W = QV = 1. 6 x 10 -19 x 1 = 1. 6 x 10 -19 J This is called “the electronvolt”, i. e. the work done on one electron as it moves through 1 volt. We can convert J into e. V by dividing by 1. 6 x 10 -19. Convert the following work functions into electronvolts: 1) Caesium – 3. 36 x 10 -19 J 2) Sodium – 3. 78 x 10 -19 J 3) Zinc – 5. 81 x 10 -19 J

Some example questions 02/11/2020 UV light is shone onto a caesium plate and electrons are emitted from the surface. If the UV light had a frequency of 300 nm and the work function of caesium is 2. 1 e. V calculate: 1. The kinetic energy of the electrons emitted in J 2. The kinetic energy in e. V 3. The average speed of the electrons

Spectra – introduction 02/11/2020

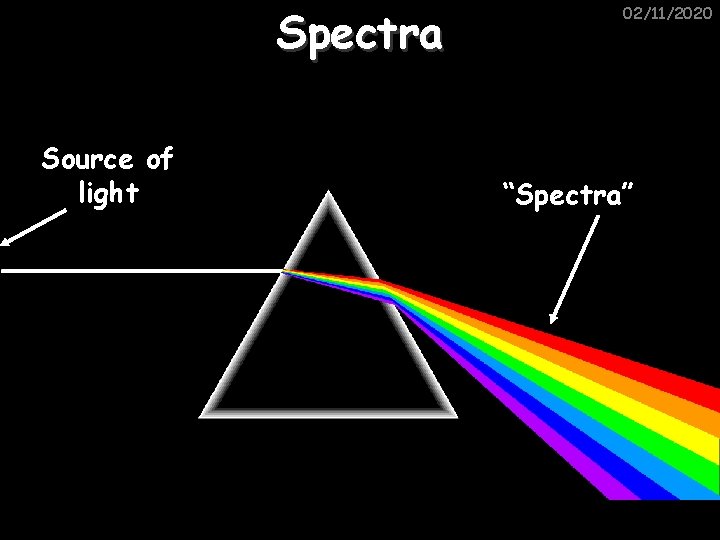
Spectra Source of light 02/11/2020 “Spectra”

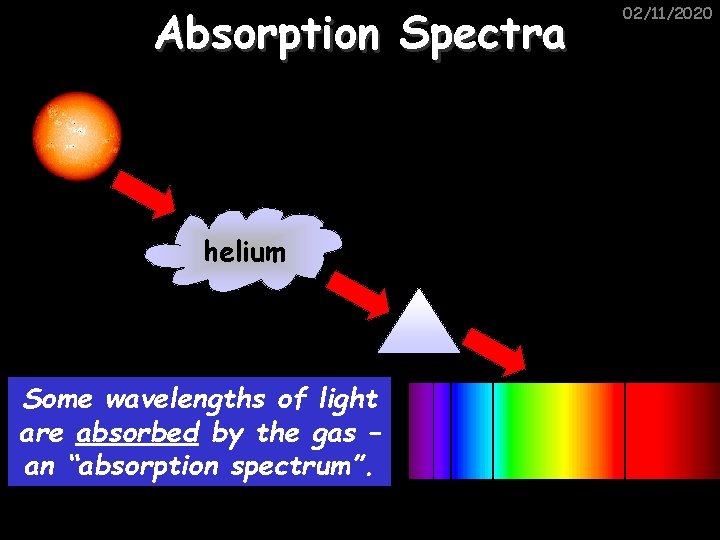
Absorption Spectra helium Some wavelengths of light are absorbed by the gas – an “absorption spectrum”. 02/11/2020

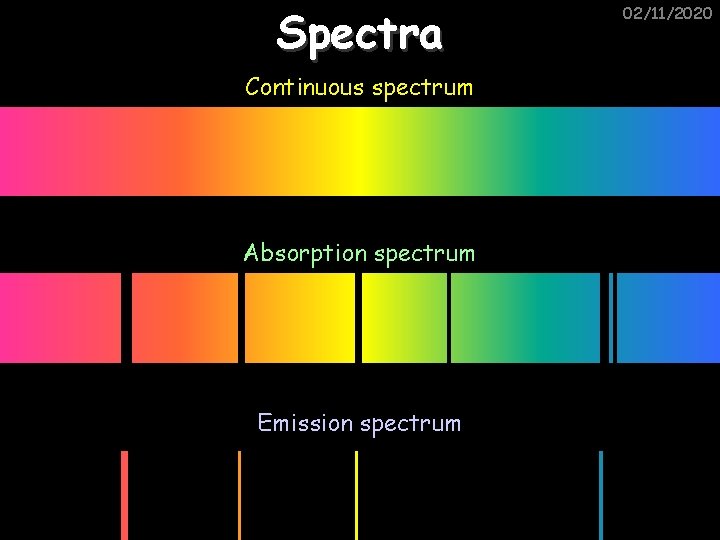
Spectra Continuous spectrum Absorption spectrum Emission spectrum 02/11/2020

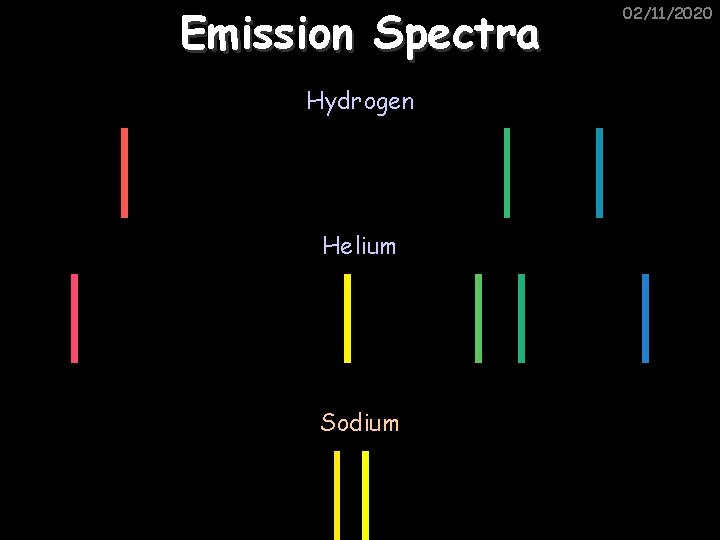
Emission Spectra Hydrogen Helium Sodium 02/11/2020

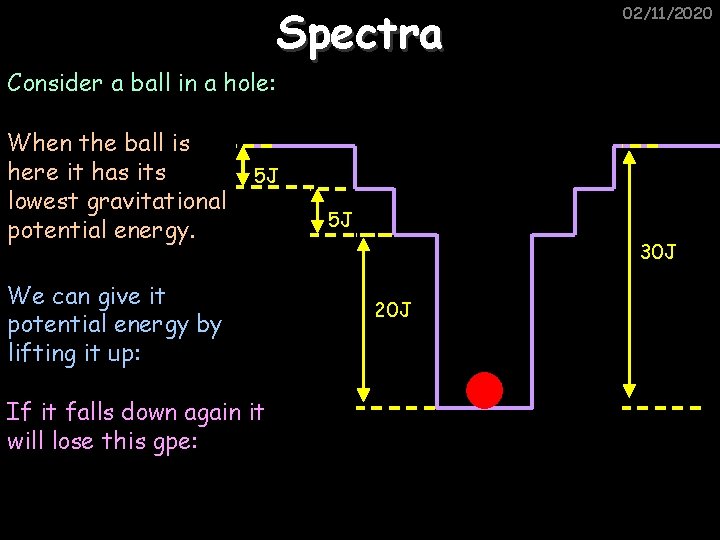
Spectra 02/11/2020 Consider a ball in a hole: When the ball is here it has its lowest gravitational potential energy. 5 J We can give it potential energy by lifting it up: If it falls down again it will lose this gpe: 5 J 30 J 20 J

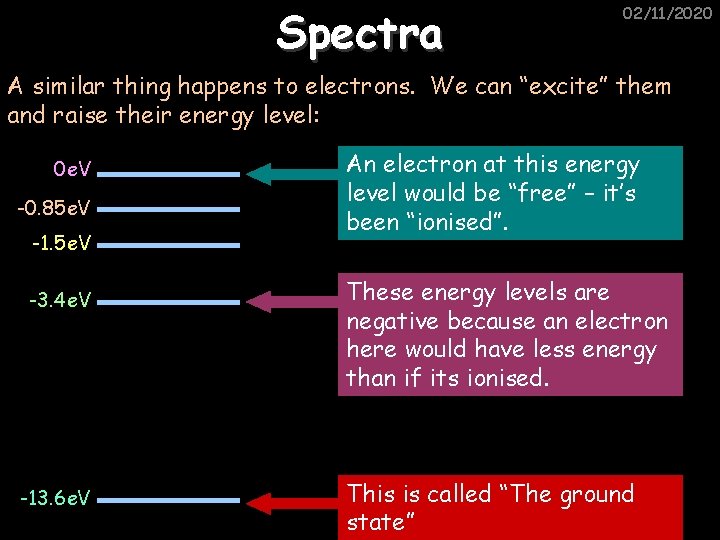
Spectra 02/11/2020 A similar thing happens to electrons. We can “excite” them and raise their energy level: 0 e. V -0. 85 e. V -1. 5 e. V -3. 4 e. V -13. 6 e. V An electron at this energy level would be “free” – it’s been “ionised”. These energy levels are negative because an electron here would have less energy than if its ionised. This is called “The ground state”

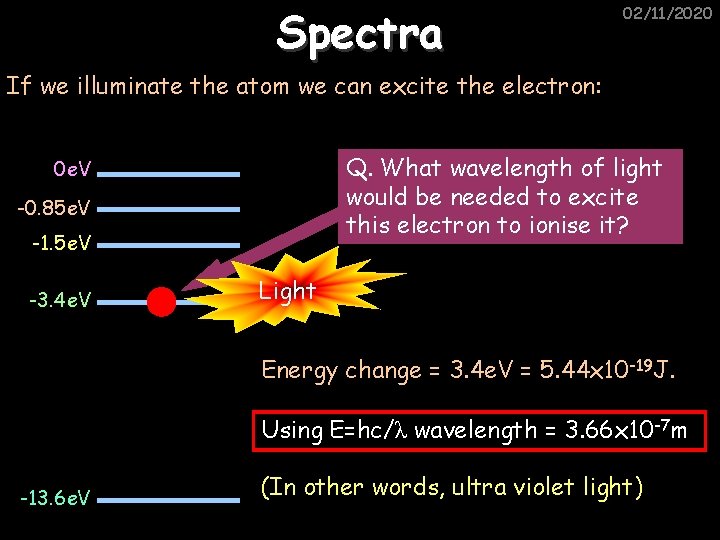
Spectra 02/11/2020 If we illuminate the atom we can excite the electron: Q. What wavelength of light would be needed to excite this electron to ionise it? 0 e. V -0. 85 e. V -1. 5 e. V -3. 4 e. V Light Energy change = 3. 4 e. V = 5. 44 x 10 -19 J. Using E=hc/λ wavelength = 3. 66 x 10 -7 m -13. 6 e. V (In other words, ultra violet light)

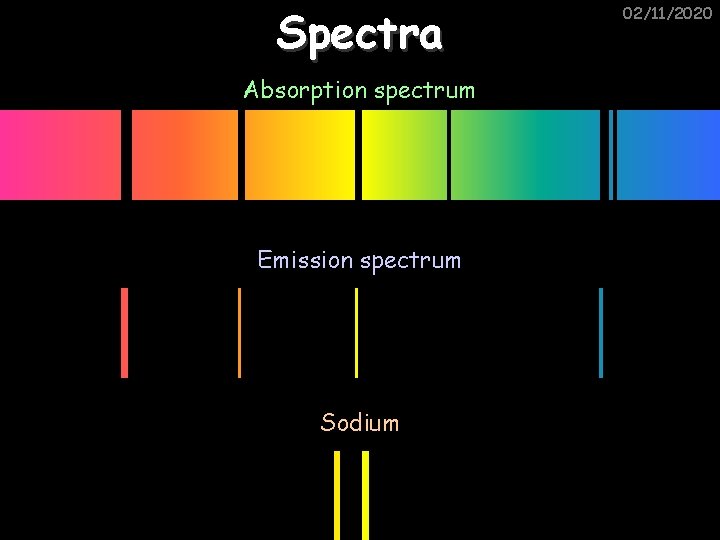
Spectra Absorption spectrum Emission spectrum Sodium 02/11/2020

Example questions 1) State the ionisation energy of this atom in e. V. 2) Calculate this ionisation energy in joules. 3) Calculate the wavelength of light needed to ionise the atom. 0 e. V -0. 85 e. V -1. 5 e. V -3. 4 e. V 4) An electron falls from the -1. 5 e. V to the -3. 4 e. V level. What wavelength of light does it emit and what is the colour? 5) Light of frequency 1 x 1014 Hz is incident upon the atom. Will it be able to ionise the atom? -13. 6 e. V 02/11/2020
 The structure of the atom section 2 defining the atom
The structure of the atom section 2 defining the atom Atom and subatomic particles
Atom and subatomic particles How are particles arranged in an atom
How are particles arranged in an atom Particles table
Particles table General arrangement of subatomic particles in the atom
General arrangement of subatomic particles in the atom The three particles that make up an atom are...
The three particles that make up an atom are... Gambar atom democritus
Gambar atom democritus Structure of atom periodic table
Structure of atom periodic table Diamond structure apf
Diamond structure apf Atom and its structure
Atom and its structure Atom and its structure
Atom and its structure Atom and its structure
Atom and its structure Atom and its structure
Atom and its structure Hát kết hợp bộ gõ cơ thể
Hát kết hợp bộ gõ cơ thể Lp html
Lp html Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Gấu đi như thế nào
Gấu đi như thế nào Chụp tư thế worms-breton
Chụp tư thế worms-breton Alleluia hat len nguoi oi
Alleluia hat len nguoi oi Các môn thể thao bắt đầu bằng tiếng chạy
Các môn thể thao bắt đầu bằng tiếng chạy Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Công của trọng lực
Công của trọng lực Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Mật thư anh em như thể tay chân
Mật thư anh em như thể tay chân 101012 bằng
101012 bằng độ dài liên kết
độ dài liên kết Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Một số thể thơ truyền thống
Một số thể thơ truyền thống Bàn tay mà dây bẩn
Bàn tay mà dây bẩn Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Thế nào là sự mỏi cơ
Thế nào là sự mỏi cơ đặc điểm cơ thể của người tối cổ
đặc điểm cơ thể của người tối cổ V. c c
V. c c Vẽ hình chiếu đứng bằng cạnh của vật thể
Vẽ hình chiếu đứng bằng cạnh của vật thể Phối cảnh
Phối cảnh Thẻ vin
Thẻ vin đại từ thay thế
đại từ thay thế điện thế nghỉ
điện thế nghỉ Tư thế ngồi viết
Tư thế ngồi viết Diễn thế sinh thái là
Diễn thế sinh thái là Các loại đột biến cấu trúc nhiễm sắc thể
Các loại đột biến cấu trúc nhiễm sắc thể Bảng số nguyên tố lớn hơn 1000
Bảng số nguyên tố lớn hơn 1000 Tư thế ngồi viết
Tư thế ngồi viết Lời thề hippocrates
Lời thề hippocrates Thiếu nhi thế giới liên hoan
Thiếu nhi thế giới liên hoan ưu thế lai là gì
ưu thế lai là gì Sự nuôi và dạy con của hổ
Sự nuôi và dạy con của hổ Khi nào hổ mẹ dạy hổ con săn mồi
Khi nào hổ mẹ dạy hổ con săn mồi Hệ hô hấp
Hệ hô hấp Từ ngữ thể hiện lòng nhân hậu
Từ ngữ thể hiện lòng nhân hậu Thế nào là mạng điện lắp đặt kiểu nổi
Thế nào là mạng điện lắp đặt kiểu nổi Describe the structure of an atom?
Describe the structure of an atom? What is fine structure of hydrogen atom
What is fine structure of hydrogen atom Section 1 structure of the atom
Section 1 structure of the atom Section 1 structure of the atom
Section 1 structure of the atom Structure of an atom
Structure of an atom Democrutus
Democrutus N in ideal gas law
N in ideal gas law Solid liquid gas particle diagram
Solid liquid gas particle diagram Properties of a solid
Properties of a solid Three subatomic particles and their charges
Three subatomic particles and their charges Neutrinous
Neutrinous