UNIT 2 ATOMIC STRUCTURE I Basic Subatomic Particles
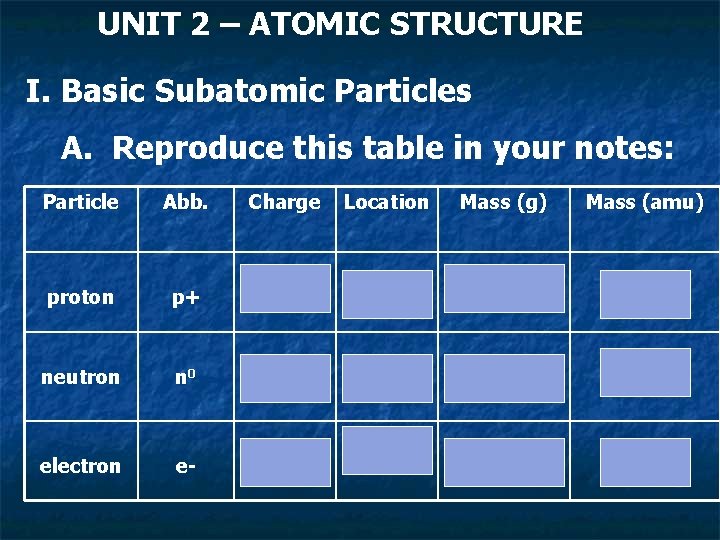
UNIT 2 – ATOMIC STRUCTURE I. Basic Subatomic Particles A. Reproduce this table in your notes: Particle Abb. Charge Location Mass (g) Mass (amu) proton p+ +1 Nucleus 1. 67 x 10 -24 ~1 amu neutron n 0 Neutral Nucleus 1. 67 x 10 -24 ~1 amu e- cloud, orbitals 9. 11 X 10 -28 1/1840 th electron e- -1

B. A typical square on the periodic table – Get out your table! 1. The Atomic Number (Z) *The whole #, the smaller of the two numbers *Corresponds to the # of p+ in an atom *Determines the identity of the atom *In an electrically neutral atom, #p+ = #e-

2. The Atomic Mass (A) *The larger of the two numbers, not usually a whole number *The mass of an atom in atomic mass units (amu), the combined mass of p+ and n 0. *What is reported on the periodic table is the weighted average of the element’s ISOTOPES. *For an individual isotope of that element: #p+ + #of n 0 = Atomic mass

C. ISOTOPES – Atoms of the same element (same # of p+, same atomic number) that have a different number of n 0 and therefore a different atomic mass. Notation #p+ #e- #n 0 Z A 1 H H-1 1 1 0 1 1 2 H H-2 1 1 2 3 H H-3 1 1 2 1 3

D. Many elements have multiple isotopes. To determine the mass seen on the periodic table the weighted average is calculated: Magnesium: Isotope Mass (amu) % Abundance Mg-24 23. 985 78. 70% Mg-25 24. 986 10. 13% Mg-26 25. 983 11. 17% Step 1: (Mass of each isotope)(% abundance as a decimal) = Step 2: Sum the calculations for all isotopes Mg-24: (23. 985)(0. 7870) = 18. 876 Mg-25 (24. 986)(0. 1013) = 2. 531 Mg-26 (25. 983)(0. 1117) = 2. 902 Weighted average is the total: 24. 309 amu
- Slides: 5